Sex-specific metabolic responses to high-fat diet in mice with NOX4 deficiency
- PMID: 40517601
- PMCID: PMC12432532
- DOI: 10.1016/j.redox.2025.103698
Sex-specific metabolic responses to high-fat diet in mice with NOX4 deficiency
Abstract
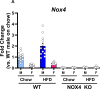
Reactive oxygen species (ROS) are critical mediators of cellular signaling that regulate metabolic homeostasis, including lipid uptake, synthesis, and storage. NADPH oxidase 4 (NOX4), a significant enzymatic source of ROS, has been identified as a redox-sensitive regulator of glucose and lipid metabolism. However, its contribution to sex-specific metabolic regulation remains poorly defined. This study compared how NOX4 knock-out (NOX4 KO) shifted systemic and tissue-specific metabolic phenotypes between male and female mice fed with a high-fat diet (HFD) for 20-weeks. We observed that male NOX4 mice on HFD exhibited reduced adiposity, diminished liver lipid accumulation, and improved glucose and insulin tolerance compared to male WT mice on HFD. In contrast, female NOX4 KO mice developed increased adiposity and lipid accumulation in peripheral adipose depots, accompanied by impaired glucose tolerance. Gene expression profiling in skeletal muscle and liver revealed distinct, sex-specific patterns of changes in genes related to lipid uptake, synthesis, and storage, possibly implicating differential activation of PPAR signaling pathways supportive of in vivo data. These findings identify NOX4 as a central regulator of sexually dimorphic lipid metabolism, acting through redox-sensitive transcriptional networks to shape divergent metabolic responses to HFD.
Copyright © 2025. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest None.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

