Efficacy and safety of drospirenone as a progestin-only pill in Japanese women: A phase III study
- PMID: 40518254
- PMCID: PMC12167661
- DOI: 10.1111/jog.16340
Efficacy and safety of drospirenone as a progestin-only pill in Japanese women: A phase III study
Abstract
Aim: To evaluate the efficacy and safety of 4 mg of drospirenone (DRSP), a progestin-only pill (POP), for contraception in Japanese women.
Methods: This was a multicenter, open-label, single-arm study. The dosing period of DRSP was 13 cycles, each lasting for 28 days. In one cycle, 4 mg of DRSP was administered orally once daily for the first 24 days, followed by a placebo for 4 days.
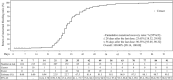
Results: Data from 276 subjects were analyzed, with a total of 3319 DRSP exposure cycles. Pregnancy occurred in one subject. The overall Pearl Index [95% CI] was 0.39 [0.01, 2.18], and the cumulative pregnancy rate [95% CI] was 0.40% [0.06, 2.81]. Of the 276 subjects, 273 (98.9%) experienced treatment-emergent adverse events (TEAEs) and 264 (95.7%) experienced adverse drug reactions. All TEAEs were mild or moderate, with no severe events. The most common TEAE was intermenstrual bleeding (irregular uterine bleeding) (89.5%). Although 31.9% of the subjects had risk factors for venous thromboembolism (VTE), no VTE-related TEAEs were observed. The incidence of unscheduled bleeding [95% CI] across all cycles was 91.6% [87.7, 94.3].
Conclusions: DRSP, the first POP in Japan, is effective and safe as a contraceptive in Japanese women. It provides a new contraceptive option for Japanese women, including those at risk of VTE for whom combined oral contraceptives are contraindicated.
Keywords: contraception; drospirenone; efficacy; progestin‐only pill; safety.
© 2025 ASKA Pharmaceutical Co., Ltd. Exeltis and The Author(s). Journal of Obstetrics and Gynaecology Research published by John Wiley & Sons Australia, Ltd on behalf of Japan Society of Obstetrics and Gynecology.
Conflict of interest statement
Kunio Kitamura received lecture fees from ASKA Pharmaceutical Co., Ltd. and Fuji Pharma Co., Ltd. Enrico Colli is an employee of Exeltis. Ryoko Kikuyama, Yumiko Kurihara, Rieko Azuma, and Tomoya Kagawa are employees of ASKA Pharmaceutical Co., Ltd.
Figures
References
-
- Osuga Y, Akiyama S, Murata T, Kidoguchi Y. Medical economic evaluation of unplanned pregnancy in Japan. J Health Care Soc. 2019;29:295–311. (in Japanese).
-
- Ministry of Health, Labor and Welfare . Overview of Health Administration Reports 2022. 2022. Available from https://www.mhlw.go.jp/toukei/saikin/hw/eisei_houkoku/22/dl/kekka5.pdf
-
- Kitamura K. SRHR is gradually becoming more widespread. Monthly J Sex Educ Today. 2024;159:1–8. (in Japanese).
-
- United Nations . Contraceptive Use by Method 2019 Data book. 2019. Available from https://digitallibrary.un.org/record/3849735?v=pdf
-
- Bonnar J. Coagulation effects of oral contraception. Am J Obstet Gynecol. 1987;157:1042–1048. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources