Histone hyperacetylation disrupts spermatogonial stem cells homeostasis and impairs spermiogenesis
- PMID: 40518506
- PMCID: PMC12168342
- DOI: 10.1186/s13287-025-04385-4
Histone hyperacetylation disrupts spermatogonial stem cells homeostasis and impairs spermiogenesis
Abstract
Background: It is well-known that epigenetic regulation is involved in the negative effects of environmental physical, chemical and biological factors exposures on organs. To investigate the effects and mechanisms of histone hyperacetylation caused by environmental stress on spermatogenesis, we used the histone deacetylase inhibitor Panobinostat (PANO) to establish the hyperacetylation models in mice.
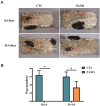
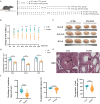
Methods: To investigate the effects of PANO on testicular function of male reproductive system, we conducted the evaluation of sperm quality parameters in mice. The morphological changes of testes were observed through hematoxylin and eosin (H&E) staining on paraffin sections and quantified by stereological measurements after treatment to explore the cause of infertility and figure out if PANO has any effect on the reproductive system of male mice. Immunofluorescence, immunohistochemistry and immunoblotting were utilized to elucidate the impact and mechanisms of PANO treatment on spermatogenesis. RNA-seq analysis was performed on mouse testes to elucidate the underlying mechanisms of PANO.
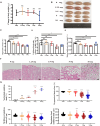
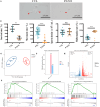
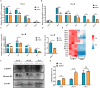
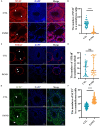
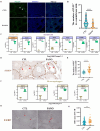
Results: The rates of sperm survival and movement were reduced while malformation rate of sperm increased in the 34.4-day PANO group. Gene Set Enrichment Analysis (GSEA) supported the significant role of PANO in modulating cilium movement, sperm axoneme assembly and flagellated sperm motility. Numbers of MVH+ cells were decreased while the numbers of SCP3+ cells were significantly increased after PANO treatment compared to those in the control (CTL) group. The protein levels of PLZF in PANO-treated testes were dramatically reduced along with the increased distribution changes of SOX9, F4/80 protein. Further data demonstrated that PANO impeded spermiogenesis at the stage XI in the 34.4-day PANO group and enhanced the transcriptome levels of histone variants H2bc4 and H1f2.
Conclusions: We have reported that PANO exerts a negative impact on the spermatogonial stem cell pool in mouse testes by disrupting its niche. This disruption leads to a reduction in germ cell numbers and impairs sperm function in mice, ultimately resulting in infertility. Moreover, PANO destabilizes the nucleosomes by increasing the transcriptional levels of H2bc4 and H1f2, affects the histone-to-protamine transition, and arrests spermiogenesis at the elongating spermatid stage. These findings also suggest that H2bc4 and H1f2 may be potential key biomarkers in the testis for diagnosing male infertility associated with aberrant histone hyperacetylation due to the exposure of environmental pollutants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study did not include clinical trials. All experiments were approved by IACUC of Nanchang University under the project “The role of activating exogenous apoptosis to remove aging hematopoietic stem cells in bone marrow reconstruction of radiation-induced chronic injury”. The approval number is NCU-CLA-2019-319. The approval date for these animal experiments is 2019-03-16. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Stage-specific embryonic antigen 4 is a membrane marker for enrichment of porcine spermatogonial stem cells.Andrology. 2020 Nov;8(6):1923-1934. doi: 10.1111/andr.12870. Epub 2020 Aug 9. Andrology. 2020. PMID: 32691968
-
Age-Dependent Clonal Expansion of Non-Sperm-Forming Spermatogonial Stem Cells in Mouse Testes.Aging Cell. 2025 Jun;24(6):e70019. doi: 10.1111/acel.70019. Epub 2025 Feb 22. Aging Cell. 2025. PMID: 39985763 Free PMC article.
-
In vivo effects of histone-deacetylase inhibitor trichostatin-A on murine spermatogenesis.J Androl. 2004 Sep-Oct;25(5):811-8. doi: 10.1002/j.1939-4640.2004.tb02859.x. J Androl. 2004. PMID: 15292114
-
[The Roles of N6-Methyladenosine Modification and Its Regulators in Male Reproduction].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):527-534. doi: 10.12182/20240560103. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948273 Free PMC article. Review. Chinese.
-
The dynamics and regulation of chromatin remodeling during spermiogenesis.Gene. 2019 Jul 20;706:201-210. doi: 10.1016/j.gene.2019.05.027. Epub 2019 May 11. Gene. 2019. PMID: 31085275 Review.
References
-
- Ou X, Yang J, Yang L et al. Histone acetylation regulated by histone deacetylases during spermatogenesis [J]. Andrology, 2024. - PubMed
-
- Liu J, Zhao M, Dong X, et al. Melatonin ameliorates PM2.5-induced spermatogenesis disorder by preserving H3K9 methylation and SIRT3 [J]. Environ Toxicol. 2024;39(3):1471–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

