Targeted inhibition of Ninjurin2 promotes chemosensitivity in chemoresistant gastric cancer by suppressing cancer-initiating cells
- PMID: 40518514
- PMCID: PMC12168268
- DOI: 10.1186/s40364-025-00792-0
Targeted inhibition of Ninjurin2 promotes chemosensitivity in chemoresistant gastric cancer by suppressing cancer-initiating cells
Abstract
Background: The combination of epirubicin, cisplatin, and 5-fluorouracil (ECF) is widely used for gastric cancer treatment. However, cancer cells can acquire chemoresistance over multiple treatment cycles, leading to recurrence. This study aimed to investigate a novel biomarker for predicting ECF resistance and its biological roles in gastric cancer.
Methods: ECF-resistant (ECF-R) gastric cancer cell lines were established through stepwise ECF treatment. Transcriptome analysis was performed to identify resistance-related genes, which were validated in tumor organoids and in vivo models. Additionally, gastric cancer patient tumor tissues were analyzed for clinical relevance.
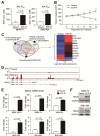
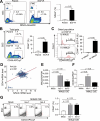
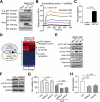
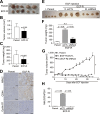
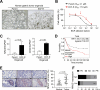
Results: Transcriptome analysis revealed that NINJURIN2 and CD44 were highly expressed in ECF-R cells but rarely expressed in normal gastric tissues. NINJURIN2 inhibition significantly increased chemosensitivity to ECF in vitro and in vivo. Liquid chromatography-tandem mass spectrometry identified periostin as a binding partner of NINJURIN2, mediating chemoresistance. Furthermore, VAV2 phosphorylation was markedly upregulated in ECF-R cells but was inhibited by NINJURIN2 knockdown. Clinical analysis showed that high NINJURIN2 expression correlated with poor survival outcomes in gastric cancer patients.
Conclusion: Our findings suggest that NINJURIN2 can be used as a novel biomarker for chemoresistant gastric cancer patients and that inhibiting NINJURIN2 along with standard chemotherapy could prevent chemoresistance-associated relapse in gastric cancer.
Keywords: Cancer initiating cells; Chemoresistance; Gastric cancer cells; Ninjurin2; Organoid.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was performed in accordance with the declaration of Helsinki. Human IHC samples were obtained from the Hospital of Yonsei University under protocols approved by the Ethics Committee. Written informed consent was obtained from each patient. Animal experiment protocols were approved by the Institutional Animal Care and Use Committee of the Yonsei Biomedical Research Institute, Yonsei University College of Medicine. Consent for publication: We have obtained consents to publish this paper from all the participants of this study. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Inhibition of EP2 receptor suppresses tumor growth and chemoresistance of gastric cancer.Am J Cancer Res. 2022 Oct 15;12(10):4680-4692. eCollection 2022. Am J Cancer Res. 2022. PMID: 36381319 Free PMC article.
-
Self-targeted knockdown of CD44 improves cisplatin sensitivity of chemoresistant non-small cell lung cancer cells.Cancer Chemother Pharmacol. 2019 Mar;83(3):399-410. doi: 10.1007/s00280-018-3737-y. Epub 2018 Dec 4. Cancer Chemother Pharmacol. 2019. PMID: 30515553
-
Efficacy and safety of docetaxel or epirubicin, combined with cisplatin and fluorouracil (DCF and ECF), regimens as first line chemotherapy for advanced gastric cancer: a retrospective analysis from Turkey.Asian Pac J Cancer Prev. 2014;15(16):6727-32. doi: 10.7314/apjcp.2014.15.16.6727. Asian Pac J Cancer Prev. 2014. PMID: 25169516 Clinical Trial.
-
Chemotherapy of oesophago-gastric cancer.Pathol Oncol Res. 1998;4(2):87-95. doi: 10.1007/BF02904700. Pathol Oncol Res. 1998. PMID: 9654592 Review.
-
[Does the FLOT regimen a new standard of perioperative chemotherapy for localized gastric cancer?].Bull Cancer. 2020 Jan;107(1):54-60. doi: 10.1016/j.bulcan.2019.12.005. Epub 2020 Jan 21. Bull Cancer. 2020. PMID: 31980145 Review. French.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Lauren PA. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

