Anxiety-like behavior during protracted morphine withdrawal is driven by gut microbial dysbiosis and attenuated with probiotic treatment
- PMID: 40518557
- PMCID: PMC12169037
- DOI: 10.1080/19490976.2025.2517838
Anxiety-like behavior during protracted morphine withdrawal is driven by gut microbial dysbiosis and attenuated with probiotic treatment
Abstract
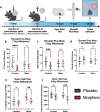
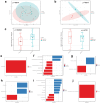
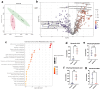
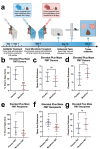
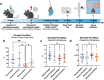
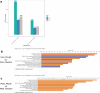
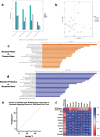
The development of anxiety during protracted opioid withdrawal heightens the risk of relapse into the cycle of addiction. Understanding the mechanisms driving anxiety during opioid withdrawal could facilitate the development of therapeutics to prevent negative affect and promote continued abstinence. Our lab has previously established the gut microbiome as a driver of various side effects of opioid use, including analgesic tolerance and somatic withdrawal symptoms. We therefore hypothesized that the gut microbiome contributes to the development of anxiety-like behavior during protracted opioid withdrawal. In this study, we first established a mouse model of protracted morphine withdrawal, characterized by anxiety-like behavior and gut microbial dysbiosis. Next, we used fecal microbiota transplantation (FMT) to show that gut dysbiosis alone is sufficient to induce anxiety-like behavior. We further demonstrated that probiotic therapy during morphine withdrawal attenuated the onset of anxiety-like behavior, highlighting its therapeutic potential. Lastly, we examined transcriptional changes in the amygdala of morphine-withdrawn mice treated with probiotics to explore mechanisms by which the gut-brain axis mediates anxiety-like behavior. Our results support the use of probiotics as a promising therapeutic strategy to prevent gut dysbiosis and associated anxiety during opioid withdrawal, with potential implications for improving treatment outcomes in opioid recovery programs.
Keywords: Opioid withdrawal; amygdala; anxiety; gut-brain axis; probiotics; serotonin.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Update of
-
Anxiety-like behavior during protracted morphine withdrawal is driven by gut microbial dysbiosis and attenuated with probiotic treatment.bioRxiv [Preprint]. 2025 Feb 1:2025.01.29.633224. doi: 10.1101/2025.01.29.633224. bioRxiv. 2025. Update in: Gut Microbes. 2025 Dec;17(1):2517838. doi: 10.1080/19490976.2025.2517838. PMID: 39975140 Free PMC article. Updated. Preprint.
Similar articles
-
Anxiety-like behavior during protracted morphine withdrawal is driven by gut microbial dysbiosis and attenuated with probiotic treatment.bioRxiv [Preprint]. 2025 Feb 1:2025.01.29.633224. doi: 10.1101/2025.01.29.633224. bioRxiv. 2025. Update in: Gut Microbes. 2025 Dec;17(1):2517838. doi: 10.1080/19490976.2025.2517838. PMID: 39975140 Free PMC article. Updated. Preprint.
-
Antibiotic treatment improves gut dysbiosis and depression-like behavior induced by morphine withdrawal.Neuropharmacology. 2025 Nov 1;278:110579. doi: 10.1016/j.neuropharm.2025.110579. Epub 2025 Jun 27. Neuropharmacology. 2025. PMID: 40582635
-
Long-lasting Depressive Behavior of Adolescent Chronically Stressed Mice is Mediated by Gut Microbiota Dysbiosis.Mol Neurobiol. 2025 Jul;62(7):8868-8886. doi: 10.1007/s12035-025-04757-0. Epub 2025 Mar 7. Mol Neurobiol. 2025. PMID: 40053245
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
Radiation-induced injury and the gut microbiota: insights from a microbial perspective.Therap Adv Gastroenterol. 2025 Jun 16;18:17562848251347347. doi: 10.1177/17562848251347347. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40535532 Free PMC article. Review.
References
-
- Bruneau A, Frimerman L, Verner M, Sirois A, Fournier C, Scott K, Perez J, Shir Y, Martel MO. Day-to-day opioid withdrawal symptoms, psychological distress, and opioid craving in patients with chronic pain prescribed opioid therapy. Drug Alcohol depend. Drug Alcohol Depen. 2021;225:108787. doi: 10.1016/j.drugalcdep.2021.108787. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
