Utilization of universal-targeting mSA2 CAR-T cells for the treatment of glioblastoma
- PMID: 40518621
- PMCID: PMC12184188
- DOI: 10.1080/2162402X.2025.2518631
Utilization of universal-targeting mSA2 CAR-T cells for the treatment of glioblastoma
Abstract
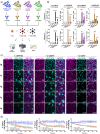
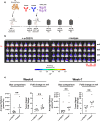
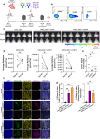
Glioblastoma (GB) remains refractory to chimeric antigen receptor (CAR)-T cell therapy, mainly attributed to tumor heterogeneity and antigen escape. CAR-T cells utilizing monomeric streptavidin-2 (mSA2) instead of a traditional target binding domain, bind biotinylated antibodies and can be directed to variable targets to mediate anti-tumor effects. Although such an approach might circumvent the aforementioned challenges, the potential of mSA2 CAR-T cells for brain tumor treatment remains unexplored. In this study, we generated mSA2 CAR-T cells and tested their efficacy against GB by tailoring their specificity toward GB-associated markers CD276, EPHA2, CD70 and IL13Ra2. In vitro, mSA2 CAR-T cells specifically recognized multiple primary GB cell lines in a target- and biotinylated antibody-dependent manner. Moreover, in heterogenous tumor environments, mSA2 CAR-T cells simultaneously targeted multiple subpopulations, guided by combinations of biotinylated antibodies, indicating their potential to address tumor heterogeneity. Finally, the mSA2 CAR-T cell-mediated anti-tumor functions were demonstrated in vivo. Immunocompromised mice orthotopically implanted with CD70+ or CD276+ GB cells and treated with mSA2 CAR-T cells pre-armed with antibodies against these two antigens exhibited control of tumor growth and induction of GB cell apoptosis after therapy. Taken together, our study suggests that antibody-guided mSA2 CAR-T cells can target potentially any surface GB-related antigen both in vitro and in vivo, either univalently or multivalently, with underlined clinical implications.
Keywords: Glioblastoma; immunotherapy; mSA2 CAR-T cells; universal antigen targeting.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





Similar articles
-
Checkpoint antibody receptor modified ARMed CAR T circumvents the suppressive immunome in GBM.Front Immunol. 2025 Jul 31;16:1579925. doi: 10.3389/fimmu.2025.1579925. eCollection 2025. Front Immunol. 2025. PMID: 40821779 Free PMC article.
-
IL-13Rα2/TGF-β bispecific CAR-T cells counter TGF-β-mediated immune suppression and potentiate anti-tumor responses in glioblastoma.Neuro Oncol. 2024 Oct 3;26(10):1850-1866. doi: 10.1093/neuonc/noae126. Neuro Oncol. 2024. PMID: 38982561
-
In vivo generation of CAR macrophages via the enucleated mesenchymal stem cell delivery system for glioblastoma therapy.Proc Natl Acad Sci U S A. 2025 Jul 22;122(29):e2426724122. doi: 10.1073/pnas.2426724122. Epub 2025 Jul 14. Proc Natl Acad Sci U S A. 2025. PMID: 40658861
-
CAR T cell therapy for glioblastoma: A review of the first decade of clinical trials.Mol Ther. 2025 Jun 4;33(6):2454-2461. doi: 10.1016/j.ymthe.2025.03.004. Epub 2025 Mar 8. Mol Ther. 2025. PMID: 40057825 Review.
-
Overcoming barriers in glioblastoma: The potential of CAR T cell immunotherapy.Theranostics. 2025 Jun 12;15(14):7090-7126. doi: 10.7150/thno.114257. eCollection 2025. Theranostics. 2025. PMID: 40585996 Free PMC article. Review.
References
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–19. doi: 10.1016/S0140-6736(20)31366-0. - DOI - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M, Lesokhin A, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
