Cucurbitacin B inhibits Th17 cell differentiation via the suppression of the JAK/STAT pathway and alleviates collagen-induced arthritis in mice
- PMID: 40518910
- PMCID: PMC12174679
- DOI: 10.1177/03946320251348715
Cucurbitacin B inhibits Th17 cell differentiation via the suppression of the JAK/STAT pathway and alleviates collagen-induced arthritis in mice
Abstract
Objective: Rheumatoid arthritis (RA) is a chronic autoimmune disease with limited treatment options and associated side effects or resistance. This study aims to investigate the therapeutic potential of the natural compound cucurbitacin B (CuB) in RA treatment.
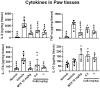
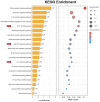
Methods: We utilized a collagen-induced arthritis (CIA) mouse model to evaluate the effects of CuB. Arthritis scores, histological damage, and pro-inflammatory cytokine expression (TNF-α, IL-17A) were assessed. In addition, network pharmacology analysis was performed to explore CuB's molecular mechanisms, focusing on Th17 cell differentiation, IL-17 signaling, and the JAK-STAT pathway.
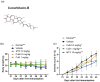
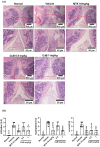
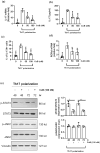
Results: CuB significantly reduced arthritis severity, decreased histological damage, and lowered the expression of pro-inflammatory cytokines in CIA mice. CuB was found to inhibit STAT3 phosphorylation and reduce the proportion of Th17 cells in the spleen, indicating its potential anti-inflammatory effects.
Conclusion: These findings suggest that cucurbitacin B may serve as a promising novel therapeutic agent for rheumatoid arthritis by targeting key inflammatory pathways.
Keywords: JAK/STAT pathway; Th17 cell differentiation; anti-inflammatory therapy; cucurbitacin B; rheumatoid arthritis (RA).
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Mechanistic insights into the anti-rheumatoid arthritis effects of ursolic acid from Periploca forrestii Schltr. via network pharmacology, molecular docking, and experimental Validation.Biochem Biophys Res Commun. 2025 Aug 15;775:152111. doi: 10.1016/j.bbrc.2025.152111. Epub 2025 May 28. Biochem Biophys Res Commun. 2025. PMID: 40466361
-
High CBD extract (CBD-X) modulates inflammation and immune cell activity in rheumatoid arthritis.Front Immunol. 2025 Jul 10;16:1599109. doi: 10.3389/fimmu.2025.1599109. eCollection 2025. Front Immunol. 2025. PMID: 40709173 Free PMC article.
-
Exploring the Targets of Gulao Yukang Pill in Rheumatoid Arthritis via the Hippo Signaling Pathway: An Integrated Network Pharmacology and Experimental Validation Study.Int J Rheum Dis. 2025 Aug;28(8):e70367. doi: 10.1111/1756-185x.70367. Int J Rheum Dis. 2025. PMID: 40725994
-
Role of the intestinal flora-immunity axis in the pathogenesis of rheumatoid arthritis-mechanisms regulating short-chain fatty acids and Th17/Treg homeostasis.Mol Biol Rep. 2025 Jun 21;52(1):617. doi: 10.1007/s11033-025-10714-w. Mol Biol Rep. 2025. PMID: 40544212 Review.
-
JAK-STAT inhibitors in noninfectious uveitis - A review.Indian J Ophthalmol. 2025 Jun 1;73(6):807-815. doi: 10.4103/IJO.IJO_61_25. Epub 2025 May 28. Indian J Ophthalmol. 2025. PMID: 40434456 Free PMC article. Review.
References
-
- Honorati MC, Meliconi R, Pulsatelli L, et al. IL-17 contributes to cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartil 2002; 10:799–807. - PubMed
-
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J Biol Chem 2003; 278:40197–40204. - PubMed
-
- Lubberts E. The IL-23–IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol 2015; 11:415–429. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

