Unlocking Chromium Decarboxylative Ligand-to-Metal Charge Transfer: Efficient and Redox-Neutral Allylation of Aldehydes Using Carboxylic Acids
- PMID: 40518937
- PMCID: PMC12232319
- DOI: 10.1021/jacs.5c04691
Unlocking Chromium Decarboxylative Ligand-to-Metal Charge Transfer: Efficient and Redox-Neutral Allylation of Aldehydes Using Carboxylic Acids
Abstract
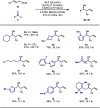
Here, we report the light-induced decarboxylative ligand-to-metal charge transfer (LMCT) of Cr(III) carboxylate complexes and demonstrate its applicability toward stereoselective Nozaki-Hiyama-Kishi (NHK) allylation reactions. The critical design element of our reaction was identifying a bipyridyl ligand scaffold that enables a single Cr catalyst to facilitate both photolytic dissociation and aldehyde addition. This approach allows for the direct utilization of carboxylic acids and eliminates the need for external redox reagents. The broad utility of this protocol was demonstrated by the preparation of a variety of homoallylic alcohols in good yields and diastereoselectivities as well as the identification of advantageous retrosynthetic disconnections. Extensive studies supported the LMCT mechanism of this transformation, including the characterization of the catalytically active Cr-carboxylate species.
Figures
References
-
-
For selected examples of reviews on decarboxylative coupling, see:
- Shang R., Liu L.. Transition metal-catalyzed decarboxylative cross-coupling reactions. Sci. China Chem. 2011;54:1670–1687. doi: 10.1007/s11426-011-4381-0. - DOI
- Rodríguez N., Goossen L. J.. Decarboxylative coupling reactions: a modern strategy for C–C-bond formation. Chem. Soc. Rev. 2011;40:5030–5048. doi: 10.1039/c1cs15093f. - DOI - PubMed
- Dzik W. I., Lange P. P., Gooßen L. J.. Carboxylates as sources of carbon nucleophiles and electrophiles: comparison of decarboxylative and decarbonylative pathways. Chem. Sci. 2012;3:2671–2678. doi: 10.1039/c2sc20312j. - DOI
- Cornella J., Larrosa I.. Decarboxylative Carbon–Carbon Bond-Forming Transformations of (Hetero)aromatic Carboxylic Acids. Synthesis. 2012;44:653–676. doi: 10.1055/s-0031-1289686. - DOI
- Laudadio G., Palkowitz M. D., Ewing T. E.-H., Baran P. S.. Decarboxylative Cross-Coupling: A Radical Tool in Medicinal Chemistry. ACS Med. Chem. Lett. 2022;13(9):1413–1420. doi: 10.1021/acsmedchemlett.2c00286. - DOI - PMC - PubMed
-
-
- Brown B. R.. The mechanism of thermal decarboxylation. Q. Rev. Chem. Soc. 1951;5:131–146. doi: 10.1039/qr9510500131. - DOI
-
-
For selected examples of reviews on radical decarboxylation protocols, see:
- Li Y., Ge L., Muhammad M. T., Bao H.. Recent Progress on Radical Decarboxylative Alkylation for Csp3–C Bond Formation. Synthesis. 2017;49(24):5263–5284. doi: 10.1055/s-0036-1590935. - DOI
- Hu X.-Q., Liu Z.-K., Hou Y.-X., Gao Y.. Single Electron Activation of Aryl Carboxylic Acids. iScience. 2020;23(7):101266. doi: 10.1016/j.isci.2020.101266. - DOI - PMC - PubMed
- Li L., Yao Y., Fu N.. Free Carboxylic Acids: The Trend of Radical Decarboxylative Functionalization. Eur. J. Org. Chem. 2023;26(21):e202300166. doi: 10.1002/ejoc.202300166. - DOI
-
-
- Tsang W.. The stability of alkyl radicals. J. Am. Chem. Soc. 1985;107(10):2872–2880. doi: 10.1021/ja00296a007. - DOI
-
- Johnson R. G., Ingham R. K.. The Degradation Of Carboxylic Acid Salts By Means Of Halogen - The Hunsdiecker Reaction. Chem. Rev. 1956;56(2):219–269. doi: 10.1021/cr50008a002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources