Integration of Bioengineered Tools in Assisted Reproductive Technologies
- PMID: 40519072
- PMCID: PMC12365630
- DOI: 10.1002/adhm.202500918
Integration of Bioengineered Tools in Assisted Reproductive Technologies
Abstract
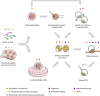
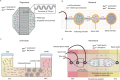
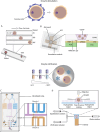
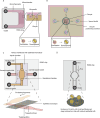
Infertility affects ≈17.5% of the adult population worldwide, posing significant clinical, emotional, and socioeconomic challenges. Recent advances at the intersection of reproductive medicine and bioengineering offer promising avenues to enhance assisted reproductive technologies (ART). This review synthesizes emerging microengineered and stem cell-based platforms designed to improve key ART stages from gamete handling and fertilization to embryo culture and implantation. State-of-the-art microfluidic systems that refine sperm selection by leveraging directional behavioral responses, enhancing motility, and preserving DNA integrity is discussed. Moreover, devices for oocyte denudation and cryopreservation have been developed to mitigate cellular stress associated with conventional processing techniques. While microengineered platforms demonstrate promise in sperm sorting and reducing stress on gametes, their broader application in ART, particularly for oocyte handling and embryo culture, requires further development. The review further addresses stem cell-based embryo models and bioengineered endometrial platforms, which aim to recapitulate the dynamic in vivo microenvironments needed for successful fertilization, embryo development, and implantation. Despite encouraging preliminary results, challenges such as scalability, reproducibility, clinical validation, and ethical considerations remain. By identifying these gaps and proposing future directions, this review considers integrating microengineering and bioengineering approaches to streamline ART procedures, ultimately enabling more personalized and effective reproductive therapies.
Keywords: assisted reproductive technologies; bioengineered platforms; fertilization; implantation; in vitro models; stem cell‐based embryo models.
© 2025 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

