Role of local complement activation in kidney fibrosis and repair
- PMID: 40519169
- PMCID: PMC12165786
- DOI: 10.1172/JCI188345
Role of local complement activation in kidney fibrosis and repair
Abstract
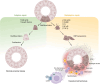
The complement system is an important component of the innate immune system involved in host defense and maintaining homeostasis. While the liver is the main source of complement proteins in the bloodstream, recent research has shown that various tissues, including the kidneys, can produce complement components locally in response to both acute and chronic inflammation. This Review highlights evidence from animal models of glomerular and tubulointerstitial kidney disease showing increased expression of intracellular complement in the kidneys. Studies using knockout mice for complement and complement receptors, along with complement inhibitors, have demonstrated that reduced complement activation in animal models of kidney fibrosis led to reduced inflammation and fibrosis, thereby supporting the pathogenic role of complement activation. Data from single-cell RNA-sequencing, spatial transcriptomics, and proteomics studies further demonstrate that alterations in local complement levels contribute to the fibrotic microenvironment observed in these models. Additionally, kidney biopsy results from patients with acute kidney injury and chronic kidney disease (CKD) indicate an increased expression of intracellular complement components as disease progresses. Developing drugs aimed at diminishing the expression and activation of local complement in glomerular and tubulointerstitial kidney disease could provide a novel approach to managing CKD.
Conflict of interest statement
Figures
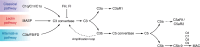

Similar articles
-
Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage.Front Immunol. 2020 May 7;11:734. doi: 10.3389/fimmu.2020.00734. eCollection 2020. Front Immunol. 2020. PMID: 32457738 Free PMC article. Review.
-
Molecules Great and Small: The Complement System.Clin J Am Soc Nephrol. 2015 Sep 4;10(9):1636-50. doi: 10.2215/CJN.06230614. Epub 2015 Jan 7. Clin J Am Soc Nephrol. 2015. PMID: 25568220 Free PMC article. Review.
-
Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis.Am J Physiol Renal Physiol. 2017 Mar 1;312(3):F516-F532. doi: 10.1152/ajprenal.00604.2016. Epub 2017 Jan 4. Am J Physiol Renal Physiol. 2017. PMID: 28052876 Free PMC article.
-
Complement Activation in Progression of Chronic Kidney Disease.Adv Exp Med Biol. 2019;1165:423-441. doi: 10.1007/978-981-13-8871-2_20. Adv Exp Med Biol. 2019. PMID: 31399977 Review.
-
Macrophages in kidney injury, inflammation, and fibrosis.Physiology (Bethesda). 2015 May;30(3):183-94. doi: 10.1152/physiol.00046.2014. Physiology (Bethesda). 2015. PMID: 25933819 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

