Disease-free survival as a surrogate for overall survival in early-stage pancreatic cancer trials: a correlation meta-analysis
- PMID: 40519225
- PMCID: PMC12164301
- DOI: 10.1136/bmjonc-2025-000766
Disease-free survival as a surrogate for overall survival in early-stage pancreatic cancer trials: a correlation meta-analysis
Abstract
Objective: Disease-free survival (DFS) is frequently used as the primary endpoint in trials of adjuvant and neoadjuvant therapies for early-stage pancreatic cancer (PC), but its validity as a surrogate for overall survival (OS) remains uncertain. This study evaluates the strength and consistency of DFS as a surrogate for OS in early-stage PC trials.
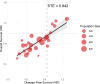
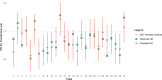
Methods and analysis: This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Trials of early-stage PC involving drug therapy were identified through PubMed, Embase and Cochrane CENTRAL databases, and data on DFS and OS with HR and CIs were extracted. Trial-level surrogacy of DFS for OS was assessed using weighted linear regression, calculating the coefficient of determination (R²) and surrogate threshold effect (STE).
Results: 29 trials involving 6777 patients were included. In adjuvant trials, HR of DFS strongly correlated with HR of OS (R²=0.70) at trial-level, with the STE of 0.94 indicating the maximum DFS HR beyond which OS benefit was unlikely. The correlation strength differed between phase III (R²=0.71) versus phase II (R²=0.67) trials. This correlation was stronger in trials including radiation therapy (R²=0.81) and trials in the neoadjuvant setting (R²=0.90). No trial in our study was a registration trial of a new molecule and all involved chemotherapy.
Conclusion: Treatment effects on DFS had a strong correlation with treatment effects on OS, making DFS a potential surrogate endpoint for OS in early-stage PC trials of cytotoxic chemotherapies, but its use in registration trials requires careful consideration due to variability across treatment settings and trial designs.
Prospero registration number: CRD42024595441.
Keywords: Adjuvant therapy; Pancreatic cancer.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
BG reports receiving consulting fees from Vivio Health and holding stock options in Onecell Diagnostics. All other authors report no relationships that could be construed as conflicts of interest for the present manuscript. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Figures

References
-
- FDA Table of surrogate endpoints that were the basis of drug approval or licensure. [17-Dec-2024]. https://www.fda.gov/drugs/development-resources/table-surrogate-endpoint... Available. Accessed.
LinkOut - more resources
Full Text Sources
Research Materials
