Multi-neuromeric origin of tyrosine hydroxylase-positive neurons within the substantia nigra and ventral tegmental area
- PMID: 40519251
- PMCID: PMC12162536
- DOI: 10.3389/fnana.2025.1612529
Multi-neuromeric origin of tyrosine hydroxylase-positive neurons within the substantia nigra and ventral tegmental area
Abstract
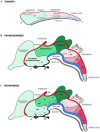
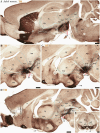
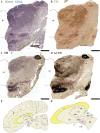
During early developmental stages, the brain is divided into three primary regions: the forebrain (prosencephalon), the hindbrain (rhombencephalon), and the spinal cord. These regions are further segmented into transverse units called neuromeres, each with distinct molecular identities that guide their specialization through development. Such modular organization is evolutionarily conserved and shapes the structural and functional complexity of the brain. The substantia nigra (SN) and ventral tegmental area (VTA) are key midbrain regions involved in reward, motivation, and motor control. They contain dopamine-producing tyrosine hydroxylase (TH)-positive neurons, which are historically classified into three anatomical groups-A8 (retrorubral field), A9 (SN pars compacta), and A10 (VTA)-each with distinct anatomical and functional properties. Recent studies revealed further sub-regional organization along medial-lateral and anterior-posterior gradients, suggesting specialized roles tied to their developmental origins. This study uses the prosomeric framework to map the segmental distribution of TH-positive neurons within the SN and VTA across different mammalian species and developmental stages. Using a comparative analysis of rodent, non-human primate and human specimens, we were able to demonstrate that TH-positive neurons within the SN and VTA exhibit a multi-neuromeric organization, with neuronal populations distributed across the diencephalic prosomeres (dp1-dp3), the midbrain prosomeres (mp1-mp2) and the isthmic rhombomere (r0). It is therefore conceivable that such multi-neuromeric origin of TH-positive neurons within the SN and VTA likely influence the patterns of connectivity and functional specialization of the dopamine system.
Keywords: SN; VTA; diencephalon; dopamine; mesomeres; nigrostriatal; prosomeres; rhombomere.
Copyright © 2025 Ferran, Lucero-Arteaga, Ayad, Kutsenko, Alonso, Do-Couto, García-Cabezas and Tseng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







Similar articles
-
Nuclear organization of the substantia nigra, ventral tegmental area and retrorubral field of the common marmoset (Callithrix jacchus): A cytoarchitectonic and TH-immunohistochemistry study.J Chem Neuroanat. 2016 Nov;77:100-109. doi: 10.1016/j.jchemneu.2016.05.010. Epub 2016 Jun 9. J Chem Neuroanat. 2016. PMID: 27292410
-
The cytoarchitectonic and TH-immunohistochemical characterization of the dopamine cell groups in the substantia nigra, ventral tegmental area and retrorubral field in a bat (Artibeus planirostris).Neurosci Res. 2016 Nov;112:37-46. doi: 10.1016/j.neures.2016.06.005. Epub 2016 Jun 24. Neurosci Res. 2016. PMID: 27349153
-
Structure of longitudinal brain zones that provide the origin for the substantia nigra and ventral tegmental area in human embryos, as revealed by cytoarchitecture and tyrosine hydroxylase, calretinin, calbindin, and GABA immunoreactions.J Comp Neurol. 2001 Jan 1;429(1):22-44. doi: 10.1002/1096-9861(20000101)429:1<22::aid-cne3>3.0.co;2-x. J Comp Neurol. 2001. PMID: 11086287
-
Reward and aversion in a heterogeneous midbrain dopamine system.Neuropharmacology. 2014 Jan;76 Pt B(0 0):351-9. doi: 10.1016/j.neuropharm.2013.03.019. Epub 2013 Apr 8. Neuropharmacology. 2014. PMID: 23578393 Free PMC article. Review.
-
The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases.Adv Anat Embryol Cell Biol. 1991;121:1-132. Adv Anat Embryol Cell Biol. 1991. PMID: 2053466 Review.
References
-
- Albuixech-Crespo B., Lopez-Blanch L., Burguera D., Maeso I., Sanchez-Arrones L., Moreno-Bravo J. A., et al. (2017). Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS Biol. 15:e2001573. doi: 10.1371/journal.pbio.2001573, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

