Use of sodium valproate and other antiseizure drug treatments in England and Wales: quantitative analysis of nationwide linked electronic health records
- PMID: 40519511
- PMCID: PMC12164316
- DOI: 10.1136/bmjmed-2023-000760
Use of sodium valproate and other antiseizure drug treatments in England and Wales: quantitative analysis of nationwide linked electronic health records
Abstract
Objective: To investigate the use of sodium valproate in England and Wales, including during pregnancy, compared with other antiseizure drug treatments, based on national level electronic health records.
Design: Quantitative analysis of nationwide linked electronic health records.
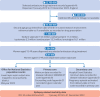
Setting: Individual level, population scale data from NHS England's Secure Data Environment, from the British Heart Foundation Data Science Centre's CVD-COVID-UK/COVID-IMPACT Consortium (for England), and the Secure Anonymised Information Linkage Databank (for Wales), 1 January 2019 to 31 December 2023.
Participants: 1 200 000 individuals dispensed any selected antiseizure drug treatment (ie, sodium valproate, lamotrigine, levetiracetam, carbamazepine, or topiramate); 304 000 women, aged 15-49 years, dispensed any selected antiseizure drug treatment and 28 400 women, aged 15-49 years, dispensed sodium valproate.
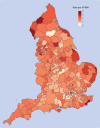
Main outcome measures: Prevalent (current) and incident (new) uses of sodium valproate and other antiseizure drug treatments before and during the covid-19 pandemic (1 January 2019 to 31 December 2023), grouped by age and sex. Pregnancy rates per 1000 women, aged 15-49 years, who used antiseizure drug treatments, and timing and dose of sodium valproate dispensed during pregnancy. Geographical variation in use of sodium valproate and disease indications (epilepsy and bipolar affective disorder). Trends in deaths related to epilepsy for 2015-22.
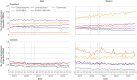
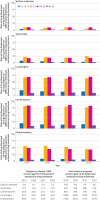
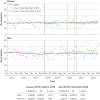
Results: Prevalent use of sodium valproate in women of childbearing potential decreased and use of most other antiseizure drug treatments increased between 2019 and 2023. Incident use of sodium valproate per 100 000 women decreased from seven to five in women aged 15-19 years, from 11 to seven in women aged 20-29 years, and from 14 to seven in women aged 30-39 years between 2019 and 2022. Incident use also decreased in men of the same age but remained at much higher levels (from 53 to 43 in men aged 15-19 years, 59 to 47 in men aged 20-29 years, and 57 to 42 in men aged 30-39 years, per 100 000 men). Pregnancy rates decreased from 6.0 to 5.2 per 1000 women of childbearing potential who were dispensed sodium valproate over the same period. The number of pregnant women who used sodium valproate during pregnancy decreased from 140 in 2019 to 85 in 2023. Epilepsy was the most common indication, followed by bipolar affective disorder (751 and 193 per 1000 women of childbearing potential dispensed sodium valproate, respectively, in 2023). No clear evidence was found that deaths related to epilepsy increased in women aged 15-49 during 2015-22, but a slight increase was found in men aged 15-49 during the later period between April 2018 and December 2022.
Conclusions: Based on comprehensive national records, changes in the dispensing of antiseizure drug treatments in response to regulatory actions were tracked. Rates for use of sodium valproate by women, including during pregnancy, decreased before and continued to slowly decrease during the covid-19 pandemic. Incident use was also reduced in men but remained at much higher levels than in women. This approach, linking national dispensing data to health records at the individual level, could help monitor changes to medicines affected by regulatory changes, including in specific population groups, such as pregnant individuals, and their potential effect on health outcomes.
Keywords: Medical informatics; Neurology; Pharmacology; Prenatal care.
Copyright © Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the British Heart Foundation Data Science Centre, Economic and Social Research Council (ESRC), Health and Care Research Wales, Engineering and Physical Sciences Research Council, Public Health Agency, Health and Social Care Research and Development Division, Alan Turing Institute, Chief Scientist Office, Scottish Government Health and Social Care Directorate, UK Research and Innovation (UKRI), Health Data Research UK, Department of Health and Social Care, Wellcome Trust, Medical Research Council (MRC), National Institute for Health and Care Research (NIHR), Administrative Data Research Wales, Health and Care Research Wales, and Medical Research Foundation for the submitted work; MP declares support paid to the University of Liverpool from Health Data Research UK, grant funding from the MRC and four industry partners (Roche, UCB, Eli Lilly, and Novartis) for the MRC Clinical Pharmacology Training Scheme, grant funding from the MRC and four industry partners (Astra Zeneca, GlaxoSmithKline (GSK), Hammersmith Medicines Research, and Optum) for the MRC Medicines Development Fellowship, developed an HLA genotyping panel with MC Diagnostics with funding from NIHR i4i (no financial benefit), IMI funding for the Accelerating Research and Development for Advanced Therapies Consortium (www.ardat.org), MP is a medical trustee for British Heart Foundation, council member for the MRC, chair of the Prix Galien Foundation UK, and chair of the Commission on Human Medicines (CHM); DH is a member of CHM; RS is a member of the Pharmacovigilance Expert Advisory Group, a subcommittee of CHM; CT has received funding paid to University College London (UCL-GSK Phenomics Hub) from GSK, outside of the scope of the submitted work; AGM has received funding paid to University of Liverpool from UCB Pharma, Sanofi, Eiasi, and GSK, he is a member of the Medicines and Healthcare products Regulatory Agency valproate implementation group, chief investigator of the Standard and New Antiepileptic Drugs (SANAD) trials, and senior author of the Cochrane reviews assessing the teratogenic effects of antiseizure drug treatments; FT declares support from UKRI-MRC-Dementias Platform UK 2-Integrated Dementia Experimental Medicine (MR/T033371/1), UKRI-ESRC, Health and Care Research Wales; KK was chair of the ethnicity subgroup of the UK Scientific Advisory Group for Emergencies (SAGE) and is a member of SAGE, and has acted as a consultant, speaker, or received grants for investigator-initiated studies for Astra Zeneca, Bayer, Novo Nordisk, Sanofi-Aventis, Servier, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, Pfizer, Roche, Daiichi-Sankyo, Applied Therapeutics, Embecta, and Nestle Health Science; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397:1375–86. doi: 10.1016/S0140-6736(21)00246-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
