A refined comparative mouse model of acute and chronic atopic dermatitis
- PMID: 40519610
- PMCID: PMC12159699
- DOI: 10.5187/jast.2024.e93
A refined comparative mouse model of acute and chronic atopic dermatitis
Abstract
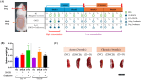
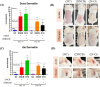
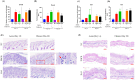
Canine and human atopic dermatitis (AD) is a complex inflammatory skin disorder with an increasing incidence, characterized by distinct acute and chronic phases with unique histological and immunological profiles. Although research into effective treatment methods has been insufficient, there has been a surge in the exploration of probiotics as a therapeutic strategy for AD. Such probiotics are often originated from the animals, and these are being developed to modulate the immune system and enhance skin barrier function, offering promising new treatment options for AD. To better understand the pathogenesis of both canine and human AD and develop treatments, animal models that accurately replicate the symptoms of both species are indispensable. This study aimed to establish a standardized and cost-effective BALB/c mouse model to more accurately simulate canine and human AD using dinitrochlorobenzene (DNCB) alone and in combination with ovalbumin (OVA). We evaluated histological and immunological changes from acute to chronic stages of AD in the mouse model induced by treatment of DNCB alone and DNCB combined with OVA to determine their similarity to both canine and human AD symptoms. The results showed that the pathological changes observed in the mouse AD model demonstrated significant parallels with both species, including increased mast cell infiltration, epidermal thickening, and elevated cytokine levels such as interleukin-4 and interferon-γ. Acute phase observations highlighted pronounced epidermal defects such as dryness and skin erosion, while chronic phase findings indicated persistent skin thickening, inflammation, and notable edema. Although both mouse models showed comparable symptoms and immunological responses, the model induced by the combination of DNCB and OVA more accurately represented canine and human AD compared to the model induced by DNCB alone. This combined DNCB and OVA mouse model provides valuable insights into AD pathogenesis and potential therapeutic targets, underscoring its significance in AD research.
Keywords: Animals; Atopic dermatitis; Mouse model; Probiotics.
© Copyright 2025 Korean Society of Animal Science and Technology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Topical application of Artemisia annua L. essential oil ameliorates 2,4-dintrochlorobenzene-induced atopic dermatitis in mice.J Ethnopharmacol. 2024 Oct 28;333:118439. doi: 10.1016/j.jep.2024.118439. Epub 2024 Jun 9. J Ethnopharmacol. 2024. PMID: 38862031
-
Ameliorative effects of Wikstroemia trichotoma 95% EtOH extract on a mouse model of DNCB-induced atopic dermatitis.J Ethnopharmacol. 2024 Oct 28;333:118398. doi: 10.1016/j.jep.2024.118398. Epub 2024 May 31. J Ethnopharmacol. 2024. PMID: 38823660
-
Sclareol attenuates the development of atopic dermatitis induced by 2,4-dinitrochlorobenzene in mice.Immunopharmacol Immunotoxicol. 2019 Feb;41(1):109-116. doi: 10.1080/08923973.2018.1555846. Epub 2019 Feb 1. Immunopharmacol Immunotoxicol. 2019. PMID: 30704333
-
Establishment and Characterization of Mild Atopic Dermatitis in the DNCB-Induced Mouse Model.Int J Mol Sci. 2023 Aug 1;24(15):12325. doi: 10.3390/ijms241512325. Int J Mol Sci. 2023. PMID: 37569701 Free PMC article.
-
FGF-21 fusion proteins ameliorate atopic dermatitis by inhibiting the TLR/TSLP signaling pathway: Anti-inflammatory and skin barrier repair effects.Int Immunopharmacol. 2025 Jun 26;159:114920. doi: 10.1016/j.intimp.2025.114920. Epub 2025 May 26. Int Immunopharmacol. 2025. PMID: 40424659 Review.
References
-
- Carretero M, Guerrero-Aspizua S, Illera N, Galvez V, Navarro M, García-García F, et al. Differential features between chronic skin inflammatory diseases revealed in skin-humanized psoriasis and atopic dermatitis mouse models. J Invest Dermatol. 2016;136:136–45. doi: 10.1038/JID.2015.362. - DOI - PubMed
LinkOut - more resources
Full Text Sources

