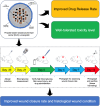
Propolis-Based Nanostructured Lipid Carrier of α-Mangostin for Promoting Diabetic Wound Healing in Alloxan-Induced Mice
- PMID: 40519653
- PMCID: PMC12165184
- DOI: 10.2147/JIR.S525243
Propolis-Based Nanostructured Lipid Carrier of α-Mangostin for Promoting Diabetic Wound Healing in Alloxan-Induced Mice
Abstract
Introduction: Diabetic wounds present a significant challenge due to delayed healing and susceptibility to infection. Conventional therapies often fall short of achieving complete and timely wound repair. This study investigates the potential of α-mangostin (αM) and its propolis-based nanostructured lipid carrier (NLC-P-αM) formulation as novel therapeutic agents for diabetic wound healing.
Purpose: To evaluate the release profile, safety, and efficacy of NLC-P-αM in promoting wound repair in an in vitro and in vivo diabetic wound model.
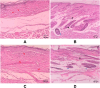
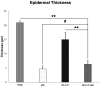
Methods: The NLC-P-αM formulation was prepared using a melt-emulsification technique with ultrasonication. In vitro release studies were conducted using a dialysis bag method and analyzed using kinetic models. Cytotoxicity was assessed using the WST-8 assay on NIH-3T3 fibroblast cells. In vivo diabetic wound healing was evaluated using alloxan-induced diabetic Swiss Webster mice. The treatments were applied topically for 14 days, and wound closure was monitored quantitatively. Histological analysis was performed to assess the inflammatory cell infiltration, epidermal thickness, and tissue regeneration.
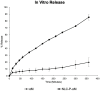
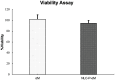
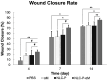
Results: NLC-P-αM demonstrated a significantly enhanced release profile, with 85.55 ± 4.25% of αM released at 360 min compared to 19.82 ± 6.78% for free αM, following a non-Fickian diffusion mechanism. Both formulations exhibited excellent safety, with cell viabilities of 94.76 ± 4.95% for NLC-P-αM and 102.16 ± 7.98% for αM in NIH-3T3 cells. In vivo, NLC-P-αM achieved the highest wound closure rate (85.83 ± 3.33%) by day 14, outperforming αM and the controls. Histological analysis confirmed reduced inflammation, a thinner epidermis, and advanced tissue regeneration in the NLC-P-αM group, highlighting its superior therapeutic efficacy.
Conclusion: NLC-P-αM demonstrated enhanced release, excellent safety, and superior efficacy in promoting diabetic wound healing compared to free αM and other controls. This nanoformulation offers a promising therapeutic strategy for accelerating wound repair in diabetic patients.
Keywords: (NLC); diabetic wound; nanostructured lipid carrier; propolis; wound healing; α-Mangostin.
© 2025 Suhandi et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Abdissa D, Adugna T, Gerema U, Dereje D. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients on follow-up clinic at Jimma medical center, Southwest Ethiopia, 2019: an institutional-based cross-sectional study. J Diabetes Res. 2020;2020:4106383. 10.1155/2020/4106383 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

