Design of a magnetic Fenton-like catalyst by decorating diamond-shaped MIL-88A with Chenopodium-derived biochar for nitrophenol degradation: optimization and mechanistic insights
- PMID: 40519682
- PMCID: PMC12164741
- DOI: 10.1039/d5ra02562a
Design of a magnetic Fenton-like catalyst by decorating diamond-shaped MIL-88A with Chenopodium-derived biochar for nitrophenol degradation: optimization and mechanistic insights
Abstract
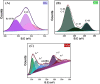
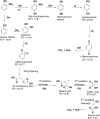
An effective Fenton-like Fe3O4/MIL-88A/BC catalyst was fabricated by combining magnetite nanoparticles (Fe3O4) with diamond-shaped MIL-88A and Chenopodium-derived biochar for the degradation of 2-NP. The elemental composition, morphology, functional groups, surface net charge, and crystallographic phase of the Fe3O4/MIL-88A/BC catalyst were examined using various characterization techniques, including XPS, SEM, FTIR, ZP, and XRD. Optimization experiments were conducted to determine the optimal Fenton-like degradation conditions for 2-NP using H2O2/Fe3O4/MIL-88A/BC. Laboratory experiments showed that the 2-NP degradation efficiency by H2O2/Fe3O4/MIL-88A/BC reached 91.04% within 120 min at pH = 5, Fe3O4/MIL-88A/BC = 10 mg, and H2O2 concentration = 500 mg L-1. Kinetic studies indicated that the Fenton-like degradation of 2-NP followed a second-order model, while H2O2 decomposition was best described by a first-order model. Quenching tests indicated that the Fenton-like reaction of 2-NP proceeded via a radical mechanism and confirmed that the ˙OH radicals are the controlling reactive O-species. The degradation mechanism of 2-NP was proposed based on the XPS spectra of the neat and used Fe3O4/MIL-88A/BC catalysts. The intermediates obtained from the Fenton-like degradation of 2-NP by the Fe3O4/MIL-88A/BC catalyst were predicted from the GC-MS spectrum.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









Similar articles
-
Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism.Nanomaterials (Basel). 2023 Dec 24;14(1):54. doi: 10.3390/nano14010054. Nanomaterials (Basel). 2023. PMID: 38202509 Free PMC article.
-
Enhanced Fenton Degradation of Tetracycline over Cerium-Doped MIL88-A/g-C3N4: Catalytic Performance and Mechanism.Nanomaterials (Basel). 2024 Jul 30;14(15):1282. doi: 10.3390/nano14151282. Nanomaterials (Basel). 2024. PMID: 39120389 Free PMC article.
-
Ingenious construction of a magnetic-recyclable photo-Fenton catalyst ZnFe2O4@MIL-88A(Fe) and its adsorption-degradation activity toward levofloxacin.J Environ Sci (China). 2025 May;151:677-691. doi: 10.1016/j.jes.2024.04.043. Epub 2024 May 7. J Environ Sci (China). 2025. PMID: 39481972
-
Core-shell structured Fe3O4@GO@MIL-100(Fe) magnetic nanoparticles as heterogeneous photo-Fenton catalyst for 2,4-dichlorophenol degradation under visible light.J Hazard Mater. 2019 Jun 5;371:677-686. doi: 10.1016/j.jhazmat.2019.03.019. Epub 2019 Mar 6. J Hazard Mater. 2019. PMID: 30889464
-
Magnetic hierarchical flower-like Fe3O4@ZIF-67/CuNiMn-LDH catalyst with enhanced redox cycle for Fenton-like degradation of Congo red: optimization and mechanism.Environ Sci Pollut Res Int. 2023 Jun;30(30):75332-75348. doi: 10.1007/s11356-023-27430-2. Epub 2023 May 23. Environ Sci Pollut Res Int. 2023. PMID: 37219772 Free PMC article.
References
-
- Marques B. S. Dalmagro K. Moreira K. S. Oliveira M. L. Jahn S. L. de Lima Burgo T. A. Dotto G. L. J. Alloys Compd. 2020;838:155628.
-
- Ewis D. Ba-Abbad M. M. Benamor A. Mahmud N. Nasser M. El-Naas M. Mohammad A. W. Int. J. Environ. Res. 2022;16:23.
-
- Khan D. Kuntail J. Sinha I. J. Mol. Graphics Modell. 2022;116:108251. - PubMed
-
- Yang Y. Gu Y. Lin H. Jie B. Zheng Z. Zhang X. J. Colloid Interface Sci. 2022;608:2884–2895. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

