A mercury complex-based fluorescent sensor for biological thiols
- PMID: 40519683
- PMCID: PMC12163708
- DOI: 10.1039/d5ra02268a
A mercury complex-based fluorescent sensor for biological thiols
Abstract
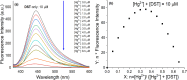
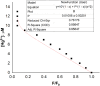
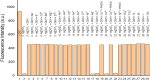
A novel fluorescent sensor, Hg(DST)2, was developed for the selective detection of biological thiols, including glutathione (GSH), cysteine (Cys), and homocysteine (Hcy), in fully aqueous solutions at pH 7.2. The sensor exhibited significant fluorescence quenching upon coordination with Hg2+, which was reversibly restored in the presence of thiols due to the formation of thermodynamically favored Hg-thiol complexes. The OFF-ON fluorescence mechanism of the sensor was elucidated using DFT calculations. Fluorescence titration experiments revealed a strong linear correlation (R 2 ≈ 0.998) between fluorescence intensity and thiol concentrations within the ranges of 0.34-8.00 μM for GSH, 0.47-10.00 μM for Cys, and 0.26-8.00 μM for Hcy, with corresponding limits of detection (LOD) of 0.34, 0.47, and 0.26 μM, respectively. The sensor demonstrated high selectivity toward thiols in the presence of common amino acids, metal ions, and anions, with interference from Ag+, Cu2+, Co2+, and Ni2+ mitigated using 1,10-phenanthroline (PHEN). Owing to its high sensitivity, selectivity, and water solubility, Hg(DST)2 represents a promising tool for thiol quantification in biological and environmental matrices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Chwatko G. Bald E. Talanta. 2000;52:509–515. - PubMed
-
- Chen D. Feng Y. Crit. Rev. Anal. Chem. 2022;52:649–666. - PubMed
-
- Nabavi S. M. and Silva A. S., Nonvitamin and Nonmineral Nutritional Supplements, Academic Press, 2018
-
- Yue Y. Huo F. Ning P. Zhang Y. Chao J. Meng X. Yin C. J. Am. Chem. Soc. 2017;139:3181–3185. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

