How to Enhance Cardiorenal Benefits in Patients With Chronic Heart Failure?
- PMID: 40519717
- PMCID: PMC12160049
- DOI: 10.36628/ijhf.2025.0004
How to Enhance Cardiorenal Benefits in Patients With Chronic Heart Failure?
Abstract
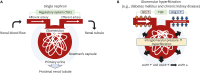
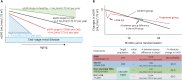
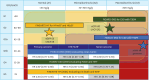
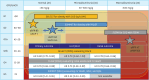
Chronic heart failure (CHF) is frequently complicated by chronic kidney disease (CKD), a comorbidity that profoundly influences disease progression, therapeutic decision-making, and clinical outcomes. The management of CHF in patients with advanced CKD presents substantial challenges, often requiring dose adjustments or even discontinuation of standard therapies. Effective therapeutic strategies must prioritize cardiorenal protection during the early stages of disease progression. Recent advancements in pharmacotherapy, including angiotensin receptor-neprilysin inhibitors, sodium-glucose cotransporter 2 inhibitors, non-steroidal mineralocorticoid receptor antagonists, and glucagon-like peptide-1 receptor agonists, have demonstrated remarkable dual cardiorenal protective effects. These therapies not only reduce the risk of de novo heart failure in high-risk populations and improve clinical outcomes in CHF patients, but also slow the progression of renal dysfunction by targeting critical pathophysiological processes, such as glomerular hyperfiltration, inflammation, ischemia, and endothelial dysfunction. Although transient declines in estimated glomerular filtration rate may occur upon initiating these agents, renal function typically stabilizes over time, facilitating sustained clinical benefits, particularly in patients with diabetes mellitus, albuminuric CKD, and CHF. This review focuses on the latest advancements in heart failure pharmacotherapy, emphasizing the cardiorenal protective mechanisms and clinical efficacy of novel therapeutic agents. It underscores the importance of bridging knowledge gaps and personalizing therapy to enhance cardiorenal benefits avoiding adverse effects.
Keywords: Cardio-renal syndrome; Cardiology; Cardiovascular diseases; Heart failure; Randomized controlled trial.
Copyright © 2025. Korean Society of Heart Failure.
Conflict of interest statement
Conflict of Interest: The authors have no financial conflicts of interest.
Figures



Similar articles
-
SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: a comprehensive review by EURECA-m and ERBP working groups of ERA.Nephrol Dial Transplant. 2023 Oct 31;38(11):2444-2455. doi: 10.1093/ndt/gfad112. Nephrol Dial Transplant. 2023. PMID: 37230946 Free PMC article. Review.
-
Combination therapy: an upcoming paradigm to improve kidney and cardiovascular outcomes in chronic kidney disease.Nephrol Dial Transplant. 2025 Feb 5;40(Supplement_1):i3-i17. doi: 10.1093/ndt/gfae212. Nephrol Dial Transplant. 2025. PMID: 39907543 Free PMC article. Review.
-
Therapeutic Effects of Heart Failure Medical Therapies on Standardized Kidney Outcomes: Comprehensive Individual Participant-Level Analysis of 6 Randomized Clinical Trials.Circulation. 2024 Dec 3;150(23):1858-1868. doi: 10.1161/CIRCULATIONAHA.124.071110. Epub 2024 Sep 1. Circulation. 2024. PMID: 39217458
-
The prevention and management of chronic kidney disease among patients with metabolic syndrome.Kidney Int. 2025 May;107(5):816-824. doi: 10.1016/j.kint.2024.12.021. Epub 2025 Feb 20. Kidney Int. 2025. PMID: 39986466 Review.
-
Slowing the Progression of Chronic Kidney Disease in Patients with Type 2 Diabetes Using Four Pillars of Therapy: The Time to Act is Now.Drugs. 2024 Nov;84(11):1337-1346. doi: 10.1007/s40265-024-02091-8. Epub 2024 Sep 11. Drugs. 2024. PMID: 39259460 Review.
References
-
- Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. - PubMed
-
- Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, et al. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73:1442–1447. - PubMed
-
- Miura M, Sakata Y, Miyata S, et al. Prognostic impact of subclinical microalbuminuria in patients with chronic heart failure. Circ J. 2014;78:2890–2898. - PubMed
-
- Ndumele CE, Rangaswami J, Chow SL, et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. 2023;148:1606–1635. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

