Estimation of PEX1-mediated Zellweger spectrum disorder births and population prevalence by population genetics modeling
- PMID: 40519747
- PMCID: PMC12166394
- DOI: 10.1016/j.gimo.2025.103431
Estimation of PEX1-mediated Zellweger spectrum disorder births and population prevalence by population genetics modeling
Abstract
Purpose: Zellweger Spectrum Disorder (ZSD) is a rare syndromic disorder characterized by impaired peroxisome assembly and function. Many cases are due to pathogenic variants in the PEX1 gene and are inherited in an autosomal recessive manner. As with many rare diseases, understanding the disease burden and scale of unmet need is challenging but required to support diagnosis, disease management, and development of therapies. We present a population-genetics-based model to estimate births and overall disease prevalence for patients in the United States, European countries, and Japan.
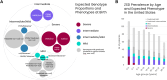
Methods: We utilized large-scale genetic diversity data sets to estimate the mutational burden per region and integrated genotype-phenotype relationships with real-world survival data to provide patient number estimates for severe, intermediate, and mild segments per age and country.
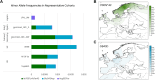
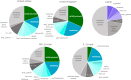
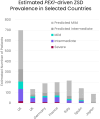
Results: We observed regional differences in the variant landscapes expected to contribute to PEX1-mediated ZSD (PEX1-ZSD). Conservative prevalence estimates for the United States, United Kingdom, Germany, France, Italy, Spain, and Japan based solely on known pathogenic variants indicates nearly 500 patients in total. Incorporating predicted pathogenic variants into our model suggests an additional 260 patients with intermediate phenotype and 930 patients with mild phenotype, under the age of 30, across these countries.
Conclusion: Notably, our model indicates that a significant proportion of patients with intermediate/mild phenotype may go unrecognized by current diagnostic practices. This diagnosis independent model of patient number estimates provides additional insights into the broad spectrum of PEX1-ZSD on a more global scale and can be used to inform health care strategies for these patients.
Keywords: Heimler syndrome; PEX1; Peroxisome biogenesis factor 1; Zellweger spectrum disorder.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
The Pex1-G844D mouse: a model for mild human Zellweger spectrum disorder.Mol Genet Metab. 2014 Apr;111(4):522-532. doi: 10.1016/j.ymgme.2014.01.008. Epub 2014 Jan 23. Mol Genet Metab. 2014. PMID: 24503136 Free PMC article.
-
Liver disease predominates in a mouse model for mild human Zellweger spectrum disorder.Biochim Biophys Acta Mol Basis Dis. 2019 Oct 1;1865(10):2774-2787. doi: 10.1016/j.bbadis.2019.06.013. Epub 2019 Jun 15. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31207289
-
Mild Zellweger syndrome due to functionally confirmed novel PEX1 variants.J Appl Genet. 2020 Feb;61(1):87-91. doi: 10.1007/s13353-019-00523-w. Epub 2019 Oct 18. J Appl Genet. 2020. PMID: 31628608 Free PMC article.
-
Diagnostic challenges and disease management in patients with a mild Zellweger spectrum disorder phenotype.Mol Genet Metab. 2021 Nov;134(3):217-222. doi: 10.1016/j.ymgme.2021.09.007. Epub 2021 Sep 27. Mol Genet Metab. 2021. PMID: 34625341 Review.
-
PEX1 mutations in the Zellweger spectrum of the peroxisome biogenesis disorders.Hum Mutat. 2005 Sep;26(3):167-75. doi: 10.1002/humu.20211. Hum Mutat. 2005. PMID: 16086329 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

