Prognostic significance of PET/CT for CAR T cell therapy in relapsed/refractory multiple myeloma
- PMID: 40519771
- PMCID: PMC12167625
- DOI: 10.1002/hem3.70159
Prognostic significance of PET/CT for CAR T cell therapy in relapsed/refractory multiple myeloma
Abstract
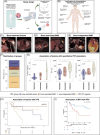
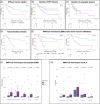
PET/CT plays an important role in staging of multiple myeloma (MM) and detecting extramedullary disease (EMD); however, its role in patients treated with commercially available CAR T cell therapies is unclear. We evaluated 61 patients treated with CAR T cell products. In 53 patients, PET/CT was available before CAR T infusion, and 43 had follow-up PET/CT on day 30. Findings from PET/CT were correlated to (CAR) T single-cell dynamics, fitness and T cell receptor diversity after infusion, and serological markers of tumor burden and inflammation. Patients with bone-independent EMD had inferior median progression-free survival (PFS: 3 vs. 15 months, p = 0.01). Univariate and multivariate analysis showed that EMD but not the number of lesions or metabolic tumor volume (MTV) were associated with inferior PFS. High MTV was connected to higher baseline sBCMA and Interleukin-6 levels, but not associated with hampered CAR T cell expansion or decreased fitness of the bystander T cell compartment. Follow-up PET/CTs identified patients with metabolic complete remissions, which were associated with better PFS. PET/CT identifies patients with high risk of relapse after CAR T cell therapy.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
M. M.: advisory boards/honoraria/research support: Amgen, BMS, Celgene, Gilead, Janssen, Stemline, Springworks, and Takeda. K. H. M.: BMS: consultancy, honoraria; AbbVie: honoraria, research funding; Pfizer: honoraria; Otsuka: honoraria; Janssen: honoraria; Novartis: consultancy. U. P.: Syros: consultancy, honoraria, research funding; MDS Foundation: membership on an entity's Board of Directors or advisory committees; Silence Therapeutics: consultancy, honoraria, research funding; Celgene: honoraria; Takeda: consultancy, honoraria, research funding; Fibrogen: research funding; Servier: consultancy, honoraria, research funding; Roche: research funding; Merck: research funding; Amgen: consultancy, research funding; Novartis: consultancy, honoraria, research funding; AbbVie: consultancy; Curis: consultancy, research funding; Janssen Biotech: consultancy, research funding; Jazz: consultancy, honoraria, research funding; BeiGene: research funding; Geron: consultancy, research funding; Bristol Myers Squibb: consultancy, honoraria, membership on an entity's Board of Directors or advisory committees; Other: travel support, medical writing support, research funding; BMS: research funding. M. J.: Novartis: honoraria; Amgen: honoraria; Pfizer: honoraria; Blueprint Medicine: honoraria; BMS: honoraria; Jazz: honoraria. The remaining authors declare no competing interests.
Figures

References
-
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328‐e346. - PubMed
-
- Hillengass J, Usmani S, Rajkumar SV, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302‐e312. - PubMed
-
- Moreau P, Attal M, Caillot D, et al. Prospective evaluation of magnetic resonance imaging and [18F]fluorodeoxyglucose positron emission tomography‐computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM Study. J Clin Oncol. 2017;35:2911‐2918. - PMC - PubMed
-
- Bailly C, Carlier T, Jamet B, et al. Interim PET analysis in first‐line therapy of multiple myeloma: prognostic value of ΔSUVmax in the FDG‐avid patients of the IMAJEM study. Clin Cancer Res. 2018;24:5219‐5224. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
