A comparative study of different assays for autoantibodies detection in patients with autoimmune gastritis
- PMID: 40519797
- PMCID: PMC12166711
- DOI: 10.1016/j.jtauto.2025.100294
A comparative study of different assays for autoantibodies detection in patients with autoimmune gastritis
Abstract
Objective: Autoimmune gastritis (AIG) is an important health problem and a risk factor for gastric neoplasms. This study assessed the diagnostic performance of different assays for anti-parietal cell antibodies (APCA) and anti-intrinsic factor antibodies (AIFA) in patients with histologically confirmed AIG.
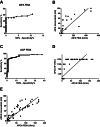
Methods: This prospective, multicenter study included 50 AIG patients and 93 controls. The diagnostic performance of fluorescent enzyme immunoassay (FEIA) and immunoblot was evaluated for the detection of both APCA and AIFA, while indirect immunofluorescence (IIF) was assessed for APCA only.
Results: Overall, AIFA detection using FEIA demonstrated slightly better performance (specificity [Sp] 100 %, positive predictive value [PPV] 100 %, negative predictive value [NPV] 75 %) compared to immunoblot (Sp 98.9 %, PPV 94.1 %, NPV 73 %). However, both methods showed low sensitivity (Se): 38 % for FEIA and 32 % for immunoblot. When the FEIA cut-off was adjusted using ROC curve analysis, Se increased to 50 %, while maintaining high Sp (98.9 %). For APCA detection, Se was similar across all methods (∼80 %), but Sp varied: immunoblot showed lower Sp (89.3 %) compared to IIF (98.8 %) and FEIA (95.7 %). PPV was highest for IIF (97.5 %), followed by FEIA (89.9 %) and immunoblot (89.3 %). NPV was lowest for immunoblot (80 %), while IIF and FEIA showed comparable values (89.5 % and 90.9 %, respectively). Adjusting the FEIA cut-off for APCA increased Sp to 98.9 % without reducing Se (76 %). Combining AIFA and APCA testing improved diagnostic performance, yielding a sensitivity of 90 % and specificity of 95.7 %.
Conclusions: FEIA offers superior diagnostic accuracy for APCA and AIFA testing in AIG. The highest diagnostic yield for AIG is observed when both APCA and AIFA are assessed. This approach could be clinically applicable in the screening for AIG and diagnostic process of AIG.
© 2025 Published by Elsevier B.V.
Conflict of interest statement
MO received travel grants from Angelini Pharma, Accord, and honoraria for lectures from Bayer, Astellas, and Sandoz, not related to the topic of this article; CH, MR, AD, NC, TMB, MVL, JCM: no conflict of interest.The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Malgorzata Osmola reports a relationship with Angelini Pharma that includes: travel reimbursement. Malgorzata Osmola reports a relationship with Accord Healthcare Inc that includes: travel reimbursement. Malgorzata Osmola reports a relationship with Bayer Corporation that includes: speaking and lecture fees. Malgorzata Osmola reports a relationship with Astellas Pharma Inc that includes: speaking and lecture fees. Malgorzata Osmola reports a relationship with Sandoz Inc that includes: speaking and lecture fees. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Coati I., Fassan M., Farinati F., Graham D.Y., Genta R.M., Rugge M. Autoimmune gastritis: pathologist's viewpoint. World J. Gastroenterol. 2015 Nov 14;21(42):12179–12189. https://pubmed.ncbi.nlm.nih.gov/26576102/ [Internet] - PMC - PubMed
-
- Lenti M.V., Rugge M., Lahner E., Miceli E., Toh B.H., Genta R.M., et al. Nature Reviews Disease Primers. Nature Research; 2020. Autoimmune gastritis. Vol. 6. - PubMed
-
- Rugge M., Bricca L., Guzzinati S., Sacchi D., Pizzi M., Savarino E., et al. Autoimmune gastritis: long-term natural history in naive Helicobacter pylori -negative patients. Gut. 2023;72(1):30–38. - PubMed
LinkOut - more resources
Full Text Sources

