GPR65 is a novel immune biomarker and regulates the immune microenvironment in lung adenocarcinoma
- PMID: 40519919
- PMCID: PMC12162609
- DOI: 10.3389/fimmu.2025.1572757
GPR65 is a novel immune biomarker and regulates the immune microenvironment in lung adenocarcinoma
Abstract
Background: The tumor microenvironment (TME) plays a crucial role in the progression of lung adenocarcinoma (LUAD), and it may serve as the best prognostic predictor of LUAD. GPR65 is an extracellular pH-sensing G protein-coupled receptor and a glycosphingolipid receptor, which is engaged in the functions of regulating tumor immunity. However, the prognostic value of GPR65 and its relevance to immune infiltration in LUAD are unknown.
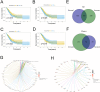
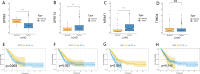
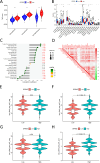
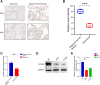
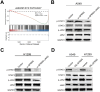
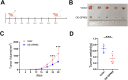
Methods: The proportion of immune, stromal and tumor cells in LUAD samples was assessed by ESTIMATE algorithm scores with RNA sequence data and clinical information from LUAD patients downloaded from The Cancer Genome Atlas (TCGA) database. We screened differential genes (DEGs) in the immune and stromal components, and then screened modular genes by the WGCNA algorithm, which were intersected with DEGs and incorporated into the LASSO-COX regression model. Additionally, nomogram containing GPR65 and clinical features were constructed for predicting patient prognosis. Then, the correlation between GPR65 and immune cell infiltration was assessed by CIBERSORT, and the impact of hub gene on immunotherapy was determined using correlation analysis between GPR65 and immune checkpoint molecules. Finally, we confirmed the expression of GPR65 in LUAD by Western Blot, Quantitative Real-time PCR and Immunohistochemistry.
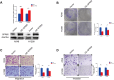
Results: In our study, we found that low expression of GPR65 was associated with poorer overall survival and primary treatment outcome in patients with LUAD. Moreover, GPR65 expression was found to be closely correlated with multiple tumor infiltrating immune cells (TIICs) and immune checkpoint molecules. Immunohistochemistry and Quantitative Real-time PCR results confirmed that the transcription levels and protein expression levels of GPR65 in LUAD tissues were significantly lower than in normal tissues. Western Blot results showed that the expression of GPR65 in human normal lung epithelial cell lines was significantly higher than the expression level in LUAD cell lines.
Conclusions: GPR65 may be an important immune biomarker in the prognosis and diagnosis of LUAD.
Keywords: Gpr65; biomarker; immune microenvironment; lung adenocarcinoma; prognosis.
Copyright © 2025 Zhou, Chen, Gao, Lian, Hu, Lu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comprehensive analysis of RFC4 as a potential biomarker for regulating the immune microenvironment and predicting immune therapy response in lung adenocarcinoma.Front Immunol. 2025 Jun 19;16:1578243. doi: 10.3389/fimmu.2025.1578243. eCollection 2025. Front Immunol. 2025. PMID: 40612960 Free PMC article.
-
Prognostic value and immune infiltration of a novel stromal/immune score-related P2RY12 in lung adenocarcinoma microenvironment.Int Immunopharmacol. 2021 Sep;98:107734. doi: 10.1016/j.intimp.2021.107734. Epub 2021 Jun 25. Int Immunopharmacol. 2021. PMID: 34175738
-
Increased expression of TTC21A in lung adenocarcinoma infers favorable prognosis and high immune infiltrating level.Int Immunopharmacol. 2020 Jan;78:106077. doi: 10.1016/j.intimp.2019.106077. Epub 2019 Dec 5. Int Immunopharmacol. 2020. PMID: 31812070
-
Profiles of immune infiltration in lung adenocarcinoma and their clinical significant: A gene-expression-based retrospective study.J Cell Biochem. 2020 Nov;121(11):4431-4439. doi: 10.1002/jcb.29667. Epub 2020 Jan 31. J Cell Biochem. 2020. PMID: 32003059
-
The role of B cell immunity in lung adenocarcinoma.Genes Immun. 2025 Jun;26(3):253-265. doi: 10.1038/s41435-025-00331-9. Epub 2025 May 13. Genes Immun. 2025. PMID: 40360749 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

