Markers of T cell activation and exhaustion in plasma are associated with persistent symptoms up to 18 months following mild SARS-CoV-2 infection
- PMID: 40519925
- PMCID: PMC12162895
- DOI: 10.3389/fimmu.2025.1578208
Markers of T cell activation and exhaustion in plasma are associated with persistent symptoms up to 18 months following mild SARS-CoV-2 infection
Abstract
Background: Persistent symptoms following SARS-CoV-2 is an increasing problem after COVID-19 disease. The pathogenesis of this persistent post Covid-19 Condition (PCC) is, however, largely unknown. We hypothesized that persistent T cell activation and exhaustion play a role in PCC development.
Methods: We examined plasma levels of soluble (s) CD25, TIM-3 and LAG-3, all markers of T cell activation/exhaustion, by enzyme immunoassays in 170 home-isolated and 53 hospitalized patients for up to 18 months after COVID-19 in relation to persistent symptomatology.
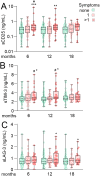
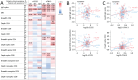
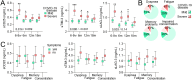
Results: Our major findings were: (i) Cases with persistent dyspnea and fatigue had markedly higher sCD25 at 6-18 months with a more modest increase in sTIM-3. (ii) Cases with memory problems at 12-18 months had increased sLAG-3 iii) sCD25 correlated with SARS-CoV-2 antibody titers and microneutralization titers only in cases with PCC while sTIM-3 correlated with these parameters irrespectively of symptoms. iv) Although hospitalized patients had markedly elevated levels of T cell activation/exhausting markers during follow-up, there was no relation to PCC symptoms.
Conclusion: Our study indicates a role for T cell activation/exhaustion in PCC following home isolated COVID-19 infection, with somewhat different patterns of sCD25, sTIM-3 and sLAG-3, but not in hospitalized COVID-19 patients where disease severity may be more important.
Keywords: SARS-CoV-2; T cell activation and exhaustion; long-term follow-up; mild covid-19 infection; persistent symptoms; post-covid condition.
Copyright © 2025 Ueland, Cox, Michelsen, Fjelltveit, Otterdal, Dahl, Zhou, Elyanow, Aukrust, Blomberg, Halvorsen and Langeland.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Figures

References
-
- World Health Organization . WHO Coronavirus (COVID-19) dashboard (2023). Available online at: https://data.who.int/dashboards/covid19/.data.who.int (Accessed March 2025).
-
- Tsilingiris D, Vallianou NG, Karampela I, Christodoulatos GS, Papavasileiou G, Petropoulou D, et al. Laboratory findings and biomarkers in long COVID: what do we know so far? Insights into epidemiology, pathogenesis, therapeutic perspectives and challenges. Int J Mol Sci. (2023) 24(13):10458. doi: 10.3390/ijms241310458 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

