Alpha-1 antitrypsin promotes re-epithelialization by regulating inflammation and migration
- PMID: 40519926
- PMCID: PMC12162329
- DOI: 10.3389/fimmu.2025.1586039
Alpha-1 antitrypsin promotes re-epithelialization by regulating inflammation and migration
Abstract
Purpose: Regulation of inflammation and re-epithelialization are critical for efficient wound healing. This study explores the role of human α1-antitrypsin (hAAT), an immunomodulatory protein, in modulating inflammation and promoting re-epithelialization across various epithelial cell types.
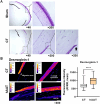
Methods: In-vitro, epithelial gap closure and migration assays were performed using two human epithelial cell lines-HaCaT and A549 cells with and without mitomycin C treatment. These cell lines were also used in an in-vitro gel-directed epithelial migration assay. Cells were treated with hAAT, and the gap area was measured using image analysis. Gene expression of inflammatory markers (IL-1β, IL-6, and TNFα) and adhesion molecules (desmoglein-1, plectin, and integrin α6β4) were analyzed using qPCR. In-vivo, corneal abrasions were induced in C57BL/6 mice using an Ophthalmic Burr. Mice received topical hAAT treatment immediately after injury and every 6 hours thereafter. Wound closure was assessed by applying the standard ophthalmic staining technique, fluorescein, and image analysis. Inflammatory markers and adhesion molecule expression were evaluated using qPCR and immunohistochemistry.
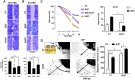
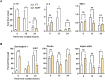
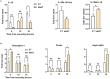
Results: In-vitro, hAAT accelerated epithelial gap closure and increased migration distance, independent of cell proliferation. hAAT-treated cells also exhibited earlier peak expressions of IL-1β and IL-6. In-vivo, hAAT treatment accelerated corneal wound closure and resulted in a preference for IL-1Ra over IL-1β expression. hAAT also enhanced the expression of desmoglein-1, plectin, and integrin α6β4, both in-vitro and in-vivo, and increased desmoglein-1 expression in the epithelial migration zone of mouse cornea.
Conclusions: hAAT enhances re-epithelialization by modulating inflammation, promoting epithelial cell migration, and regulating expression of adhesion molecules.
Keywords: adhesion molecules; corneal abrasion; desmosomes; gene expression; hemidesmosomes; inflammation; wound Healing.
Copyright © 2025 Farber, Wated, Schuster, Sheffer, Anav, Ogen-Shtern, Tsitrina, Tacruri, Bunin, Nagar, Hagbi Bal, Eliyahu, Halpern, Knyazer, Cohen, El-Saied, Lewis, Silberstein and Tsumi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Effects of quercetin on human oral keratinocytes during re-epithelialization: An in vitro study.Arch Oral Biol. 2018 Nov;95:187-194. doi: 10.1016/j.archoralbio.2018.08.004. Epub 2018 Aug 15. Arch Oral Biol. 2018. PMID: 30130672
-
Accelerated Wound Border Closure Using a Microemulsion Containing Non-Inhibitory Recombinant α1-Antitrypsin.Int J Mol Sci. 2022 Jul 1;23(13):7364. doi: 10.3390/ijms23137364. Int J Mol Sci. 2022. PMID: 35806370 Free PMC article.
-
Human Parathyroid Hormone Analog (3-34/29-34) promotes wound re-epithelialization through inducing keratinocyte migration and epithelial-mesenchymal transition via PTHR1-PI3K/AKT activation.Cell Commun Signal. 2023 Aug 23;21(1):217. doi: 10.1186/s12964-023-01243-9. Cell Commun Signal. 2023. PMID: 37612710 Free PMC article.
-
IL-20 promotes epithelial healing of the injured mouse cornea.Exp Eye Res. 2017 Jan;154:22-29. doi: 10.1016/j.exer.2016.11.006. Epub 2016 Nov 3. Exp Eye Res. 2017. PMID: 27818315 Free PMC article.
-
Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization.Tissue Eng Part B Rev. 2022 Jun;28(3):665-676. doi: 10.1089/ten.TEB.2021.0030. Epub 2021 Oct 18. Tissue Eng Part B Rev. 2022. PMID: 34238035 Review.
References
-
- Sivamani K, Shirakawa Garcia M, Rivkah Isseroff R. Cell attachments: desmosomes, hemidesmosomes, integrins 4. Keratins 5. Growth factors and cytokines 5.1. EGF family 5.2. FGF family 5.3. TGF-Beta Family 5. (2007) 12:2849–68. doi: 10.2741/2277 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

