Influence of Mucoadhesive Polymers on Physicochemical Features and Biocompatibility of Collagen Wafers
- PMID: 40519946
- PMCID: PMC12163946
- DOI: 10.1021/acspolymersau.5c00010
Influence of Mucoadhesive Polymers on Physicochemical Features and Biocompatibility of Collagen Wafers
Abstract
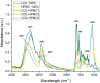
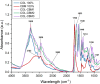
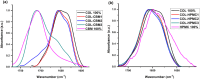
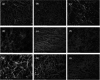
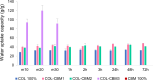
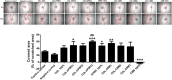
The aim of this study was to develop and characterize some freeze-dried wafers based on collagen and two mucoadhesive polymers, namely, hydroxypropyl methylcellulose (HPMC) and Carbomer 940 (CBM). The wafers were obtained by lyophilization of the corresponding hydrogels, which were evaluated by circular dichroism in order to investigate mucoadhesive polymers' influence on collagen's secondary structure. The obtained freeze-dried wafers were characterized by FT-IR spectroscopy, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), contact angle measurements, and water uptake capacity. Furthermore, biocompatibility assessment was performed by evaluating the impact of freeze-dried wafer extracts on cell viability, morphology, and migration capacity. Circular dichroism showed more significant changes in the secondary structure of collagen associated with the addition of Carbomer 940. The FT-IR spectra displayed specific peaks for collagen and the two mucoadhesive polymers. SEM images illustrated a microporous structure for both collagen and Carbomer 940, while HPMC displayed a more sheet-like structure. The addition of HPMC increased the thermal stability of collagen, while Carbomer 940 had a negative impact on the samples' thermal stability. Contact angle measurements and water uptake capacity showed good hydrophilicity of the wafers. Except for CBM 100%, all samples supported the viability of human fibroblasts and did not have any inhibitory effect on cell migration capacity, demonstrating good biocompatibility, which is an essential attribute in developing drug delivery supports intended for mucosal applications.
Keywords: biocompatibility; carbomer; collagen; hydroxypropyl methylcellulose; wafer.
© 2025 The Authors. Published by American Chemical Society.
Figures



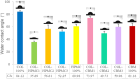




Similar articles
-
Fabrication, in Vitro, and in Vivo Characterization of Mucoadhesive Berberine-Loaded Blended Wafers for Treatment of Chemotherapy-Induced Oral Mucositis.AAPS PharmSciTech. 2022 Dec 16;24(1):19. doi: 10.1208/s12249-022-02476-6. AAPS PharmSciTech. 2022. PMID: 36526920
-
Development and functional characterization of composite freeze dried wafers for potential delivery of low dose aspirin for elderly people with dysphagia.Int J Pharm. 2018 Dec 20;553(1-2):65-83. doi: 10.1016/j.ijpharm.2018.10.025. Epub 2018 Oct 9. Int J Pharm. 2018. PMID: 30312748
-
Role of physicochemical properties of some grades of hydroxypropyl methylcellulose on in vitro mucoadhesion.Int J Pharm. 2021 Nov 20;609:121218. doi: 10.1016/j.ijpharm.2021.121218. Epub 2021 Oct 20. Int J Pharm. 2021. PMID: 34687813
-
Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations.Eur J Pharm Sci. 2021 Apr 1;159:105736. doi: 10.1016/j.ejps.2021.105736. Epub 2021 Jan 28. Eur J Pharm Sci. 2021. PMID: 33516807 Review.
-
Thermal analysis of hydroxypropylmethylcellulose and methylcellulose: powders, gels and matrix tablets.Int J Pharm. 1999 Mar 15;179(2):209-28. doi: 10.1016/s0378-5173(98)00339-1. Int J Pharm. 1999. PMID: 10053214 Review.
References
-
- Zhang, X. ; Mao, S. . Recent Development in Mucosal Drug Delivery System. In Novel Formulations and Future Trends; Academic Press, 2024; Chapter 3, pp 61–84.
-
- Pandey P., Pal R., Singh S., Gupta H., Batham A., Kumar N., Sharma A.. The Current Status in Mucosal Drug Delivery Systems (MDDS) and Future Prospectus in Delivery: A Systematic Review. Int. J. Pharm. Sci. Med. 2023;8:76–106. doi: 10.47760/ijpsm.2023.v08i10.007. - DOI
-
- Abhang P., Momin M., Inamdar M., Kar S.. Transmucosal Drug Delivery- An Overview. Drug Delivery Lett. 2014;4:26–37. doi: 10.2174/22103031113039990011. - DOI
-
- Carvalho F. C., Bruschi M., Evangelista R., Gremiao M.. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010;46:1–17. doi: 10.1590/S1984-82502010000100002. - DOI
LinkOut - more resources
Full Text Sources
