Unfolded von Willebrand factor binds protein S and reduces anticoagulant activity
- PMID: 40519989
- PMCID: PMC12162048
- DOI: 10.1016/j.bvth.2024.100030
Unfolded von Willebrand factor binds protein S and reduces anticoagulant activity
Abstract
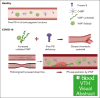
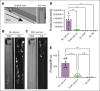
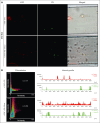
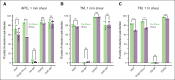
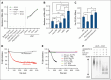
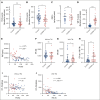
The critical plasma anticoagulant protein S (PS) circulates in 2 functionally distinct pools: free (anticoagulant) or bound to complement component 4b-binding protein (C4BP; anti-inflammatory). Acquired free PS deficiency is detected in several viral infections, but its cause is unclear. Here, we used biochemical approaches and human patient plasma samples to identify an interaction between PS and von Willebrand factor (VWF), which causes free PS deficiency and reduced PS anticoagulant activity. We first identified a shear-dependent interaction between PS and VWF by mass spectrometry. Consistently, PS and VWF could be crosslinked together in plasma, and plasma PS and VWF comigrated in gel electrophoresis. The PS/VWF interaction was blocked by and tissue factor pathway inhibitor but not activated protein C, suggesting an interaction with the sex hormone binding globulin region of PS. Microfluidic systems demonstrated that PS stably binds VWF as VWF unfolds under turbulent flow. PS/VWF complexes also localized to platelet thrombi under laminar arterial flow. In thrombin generation-based assays, shearing plasma decreased PS activity, an effect not seen in the absence of VWF. Finally, free PS deficiency in patients with COVID-19 correlated with changes in VWF, but not C4BP, and with thrombin generation. Our data indicate that PS binds to a shear-exposed site on VWF, thus sequestering free PS and decreasing its anticoagulant activity, which would account for the increased thrombin generation potential. Because many viral infections present with free PS deficiency, elevated circulating VWF, and increased vascular shear, we propose that the PS/VWF interaction reported here is a likely contributor to virus-associated thrombotic risk.
Conflict of interest statement
Conflict-of-interest disclosure: J.P.W. received an investigator-initiated grant through Pfizer Inc, unrelated to this project. The remaining authors declare no competing financial interests.
Figures


Update of
-
Unfolded Von Willebrand Factor Binds Protein S and Reduces Anticoagulant Activity.bioRxiv [Preprint]. 2024 Feb 9:2024.02.08.579463. doi: 10.1101/2024.02.08.579463. bioRxiv. 2024. Update in: Blood Vessel Thromb Hemost. 2025 Feb;2(1):100030. doi: 10.1016/j.bvth.2024.100030. PMID: 38370737 Free PMC article. Updated. Preprint.
References
-
- Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76(1):1–16. - PubMed
-
- Mahasandana C, Suvatte V, Marlar RA, Manco-Johnson MJ, Jacobson LJ, Hathaway WE. Neonatal purpura fulminans associated with homozygous protein S deficiency. Lancet. 1990;335(8680):61–62. - PubMed
-
- Walker FJ. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J Biol Chem. 1981;256(21):11128–11131. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
