Heart failure, inflammation and exercise
- PMID: 40520009
- PMCID: PMC12160080
- DOI: 10.7150/ijbs.109917
Heart failure, inflammation and exercise
Abstract
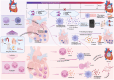
Heart failure (HF) is a condition characterized by high morbidity, mortality, and a substantial healthcare burden, in which inflammation plays a pivotal role. This review provides a comprehensive overview of inflammation in HF progression, highlighting the dynamic alterations in immune cell populations-such as monocytes/macrophages and neutrophils-and regulatory mechanisms of key signaling pathways, including JAK and NLRP3. Furthermore, the clinical relevance of inflammatory biomarkers in predicting disease prognosis is also discussed. Emerging evidence indicates that exercise intervention can enhance cardiac function by promoting the expression of anti-inflammatory cytokines (e.g., IL-10) and mitigating myocardial fibrosis, oxidative stress, and apoptosis. Future studies should investigate how exercise modulates critical inflammatory pathways-such as TLR/MyD88/NF-κB and the NLRP3 inflammasome-and aim to establish personalized exercise protocols tailored to patients' inflammatory profiles and disease stages. Such insights may pave the way for innovative therapeutic strategies in HF management.
Keywords: exercise; heart failure; inflammation; signaling pathway.
© The author(s).
Conflict of interest statement
Competing interests: The authors have declared that no competing interest exists.
Figures




References
-
- Khan MS, Shahid I, Bennis A, Rakisheva A, Metra M, Butler J. Global epidemiology of heart failure. Nature reviews Cardiology. 2024;21:717–34. - PubMed
-
- Poelzl G, Fetz B, Altenberger J, Fritsch M, Auer J, Stachl E. et al. Heart failure disease management programs in Austria 2019: A systematic survey of the Heart Failure Working Group and the Working Group for Cardiological Assistance and Care Personnel of the Austrian Society of Cardiology. Wien Klin Wochenschr. 2020;132:310–21. - PMC - PubMed
-
- Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1324–40. - PubMed
-
- Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M. et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. European Journal of Heart Failure. 2018;20:445–59. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

