Regulatory Action of Calcium and Calcium Channels in Pain Pathways
- PMID: 40520015
- PMCID: PMC12160918
- DOI: 10.7150/ijbs.110504
Regulatory Action of Calcium and Calcium Channels in Pain Pathways
Abstract
Calcium ions (Ca2+) and Ca2+ channels are pivotal in the regulation of pain pathways and serve as key regulators of neuronal excitability and neurotransmitter release. We review the different types of Ca2+ channels involved in pain processing, including voltage-gated Ca2+ channels (VGCCs), such as L-, N-, P/Q-, and T-type channels. Each subtype is intricately involved in different aspects of pain perception, from acute pain signaling to the development and maintenance of chronic pain states. In addition, the roles of transient receptor potential (TRP) channels, particularly TRPV1 and TRPA1, are discussed in the context of their contribution to chronic pain. Advances in Ca2+ imaging techniques, particularly through genetically encoded Ca2+ indicators (GECIs), such as GCaMPs, have provided unprecedented insight into the dynamic role of Ca2+ channels in pain pathways. These efforts have deepened our understanding of Ca2+ channels and suggest novel therapeutic targets for more effective pain management strategies within Ca2+ channels.
Keywords: Ca2+, Ca2+ channel; GCaMP; GECI; TRP channel; VGCC; pain; regulatory mechanism.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
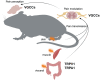
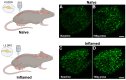
References
-
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R. et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11) Pain. 2019;160:19–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

