MNX1-AS1 suppresses chemosensitivity by activating the PI3K/AKT pathway in breast cancer
- PMID: 40520020
- PMCID: PMC12160916
- DOI: 10.7150/ijbs.104483
MNX1-AS1 suppresses chemosensitivity by activating the PI3K/AKT pathway in breast cancer
Abstract
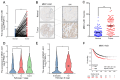
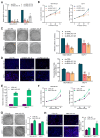
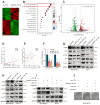
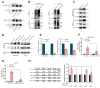
Long noncoding RNAs (lncRNAs) critically regulate tumorigenesis and chemosensitivity. Despite the pivotal role of lncRNAs in breast cancer (BC), their specific functions and underlying mechanism, particularly in the context of drug resistance, remain largely unexplored. We discovered that MNX1-AS1 is significantly elevated in BC and contributes to paclitaxel resistance through the PI3K/AKT pathway. Moreover, elevated MNX1-AS1 expression exhibits close association with unfavourable prognosis in BC. Mechanistically, MNX1-AS1 interacts with YBX1, preventing its SMURF2-mediated ubiquitination and subsequent degradation, thereby increasing YBX1 protein levels. Upregulated YBX1 transcriptionally activates the expression of ITGA6 by binding to its promoter in the nucleus. Furthermore, MNX1-AS1 binds to IGF2BP2, promoting the stability of ITGA6 mRNA in an m6A-dependent manner within the cytoplasm. MNX1-AS1 increases ITGA6 expression at transcriptional and post-transcriptional levels, thereby activating the PI3K/AKT pathway. Notably, lipid nanoparticles were implicated to effectively deliver MNX1-AS1 siRNA to tumor-bearing mice, resulting in significant antitumor effects. These findings underscore the role of MNX1-AS1 in activating the ITGA6/PI3K/AKT pathway, which facilitates tumor progression and induces chemoresistance in BC. Targeting MNX1-AS1 may represent a promosing therapeutic strategy to enhance chemotherapy efficacy in BC patients.
Keywords: ITGA6/PI3K/AKT pathway; LncRNA; breast cancer; chemosensitivity; lipid nanoparticles.
© The author(s).
Conflict of interest statement
Competing interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
YBX1/lncRNA SBF2-AS1 interaction regulates proliferation and tamoxifen sensitivity via PI3K/AKT/MTOR signaling in breast cancer cells.Mol Biol Rep. 2023 Apr;50(4):3413-3428. doi: 10.1007/s11033-023-08308-5. Epub 2023 Feb 8. Mol Biol Rep. 2023. PMID: 36754932
-
MYC-Activated LncRNA MNX1-AS1 Promotes the Progression of Colorectal Cancer by Stabilizing YB1.Cancer Res. 2021 May 15;81(10):2636-2650. doi: 10.1158/0008-5472.CAN-20-3747. Epub 2021 Mar 29. Cancer Res. 2021. PMID: 33782099
-
FOXD1-AS1 regulates FOXD1 translation and promotes gastric cancer progression and chemoresistance by activating the PI3K/AKT/mTOR pathway.Mol Oncol. 2021 Jan;15(1):299-316. doi: 10.1002/1878-0261.12728. Epub 2020 Nov 14. Mol Oncol. 2021. PMID: 32460412 Free PMC article.
-
Exosomal lncRNA FOXD3-AS1 upregulates ELAVL1 expression and activates PI3K/Akt pathway to enhance lung cancer cell proliferation, invasion, and 5-fluorouracil resistance.Acta Biochim Biophys Sin (Shanghai). 2021 Nov 10;53(11):1484-1494. doi: 10.1093/abbs/gmab129. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34605863
-
LncRNA HIF1A-AS1 Promotes Gemcitabine Resistance of Pancreatic Cancer by Enhancing Glycolysis through Modulating the AKT/YB1/HIF1α Pathway.Cancer Res. 2021 Nov 15;81(22):5678-5691. doi: 10.1158/0008-5472.CAN-21-0281. Epub 2021 Sep 30. Cancer Res. 2021. PMID: 34593522
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J Clin. 2021;71:209–49. - PubMed
-
- Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–12. - PubMed
-
- Claessens A, Ibragimova K, Geurts S, Bos M, Erdkamp F, Tjan-Heijnen V. The role of chemotherapy in treatment of advanced breast cancer: an overview for clinical practice. Crit Rev Oncol Hemat. 2020;153:102988. - PubMed
-
- Santa-Maria CA, Nanda R. Immune Checkpoint Inhibitor Therapy in Breast Cancer. J Natl Compr Canc Ne. 2018;16:1259–68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

