Liposomal Nanoparticle Delivery of Ginkgo Flavone Glycosides Enhances SIRT1 Activation and Improves Diabetic Cardiomyopathy
- PMID: 40520056
- PMCID: PMC12165216
- DOI: 10.2147/IJN.S493862
Liposomal Nanoparticle Delivery of Ginkgo Flavone Glycosides Enhances SIRT1 Activation and Improves Diabetic Cardiomyopathy
Abstract
Purpose: This study aims to explore the therapeutic mechanisms of Ginkgo Flavone Glycosides (GFGs) delivered via liposomal nanoparticles in treating Diabetic Cardiomyopathy (DCM) by upregulating Sirtuin 1 (SIRT1) to restore energy metabolism and autophagy homeostasis.
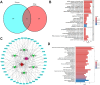
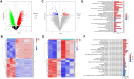
Methods: A DCM mouse model was employed, with groups treated with different doses of GFGs. Various evaluations, including body weight, blood glucose levels, and cardiac function, were performed. Network pharmacology, transcriptomic analysis, and molecular docking studies were conducted to elucidate the key role of SIRT1 in inhibiting DCM progression. In vitro experiments and proteomic sequencing were utilized to validate the regulatory effects of SIRT1.
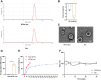
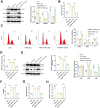
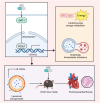
Results: The in vivo animal experiment results demonstrated that treatment with Ginkgo Flavone Glycosides (GFGs) significantly improved cardiac function in diabetic cardiomyopathy mice. Specifically, GFG treatment increased the left ventricular ejection fraction (LVEF) by approximately 81.3% compared to the Model+Lipo group, reduced the left ventricular internal diameter in systole (LVIDs) by approximately 69.2%, and decreased the left ventricular internal diameter in diastole (LVIDd) thickness by approximately 56.1%. Additionally, GFGs alleviated cardiomyocyte apoptosis, further supporting their therapeutic potential for diabetic cardiomyopathy. Bioinformatics analysis supported the regulation of DCM through the SIRT1/FOSL1/TSPAN4 axis. Proteomic data confirmed the beneficial effects of GFGs on diabetic cardiac energy metabolism and autophagy. Liposomal nanoparticles loaded with GFGs significantly extended drug release to 72 hours. In vitro experiments highlighted the role of SIRT1 in modulating FOSL1 and TSPAN4 expression. Proteomic sequencing further validated the regulatory role of the SIRT1/FOSL1/TSPAN4 signaling pathway in DCM and suggested that GFGs might enhance energy metabolism and autophagy in diabetic hearts by activating SIRT1.
Conclusion: Liposomal nanoparticle delivery of GFGs was shown to enhance SIRT1 activation, leading to the deacetylation of FOSL1 and suppression of TSPAN4, ultimately improving energy metabolism and autophagy in DCM. This study introduces a novel potential strategy for the treatment of DCM.
Keywords: Ginkgo Flavone Glycosides; Sirtuin 1; autophagy homeostasis; diabetic cardiomyopathy; energy metabolism; liposomal nanoparticles.
© 2025 Gao et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures







References
-
- Tang Y, Feng M, Su Y, et al. Jmjd4 facilitates Pkm2 degradation in cardiomyocytes and is protective against dilated cardiomyopathy [published correction appears in Circulation. 2024 Jul 9;150(2):e64. doi: 10.1161/CIR.0000000000001268]. Circulation. 2023;147(22):1684–1704. doi: 10.1161/CIRCULATIONAHA.123.064121 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

