Cerebral arteritis in bacterial meningitis: Structural adaptations of the vascular wall in response to an infectious nidus - A narrative review
- PMID: 40520064
- PMCID: PMC12164788
- DOI: 10.4103/bc.bc_4_23
Cerebral arteritis in bacterial meningitis: Structural adaptations of the vascular wall in response to an infectious nidus - A narrative review
Abstract
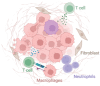
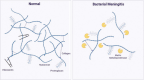
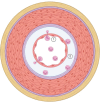
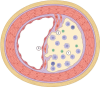
Despite dramatic improvements in diagnosis and antimicrobial treatment of bacterial meningitis over the last few decades, adverse postinfective sequelae and mortality remain exceedingly high in adults. Of note, the unfavorable clinical outcome is usually attributable to the presence of intracranial complications during the acute phase of infection, such as cerebral edema and increases in intracranial pressure and cerebral blood flow disturbances. Involvement of the cerebral vasculature during bacterial meningitis is overwhelmingly clear from clinical and laboratory evidence highlighting cerebral blood flow alterations with the use of Doppler blood flow analysis, angiographic studies of cerebral vessel wall structural irregularities and computed tomography/magnetic resonance imaging recording of cerebral infarctions. With the widespread agreement of cerebrovascular involvement in bacterial meningitis, very few studies have documented histopathological observations of cerebral vessel irregularities affecting the various layers of the vascular wall. In an attempt to understand the arterial wall changes that take place before the occurrence of cerebral ischemic consequences in bacterial meningitis, we have investigated the sequential changes affecting the arterial vasculature, beginning with early reflexive modifications of the adventitia and culminating in late proliferative lesions of the intima.
Keywords: Bacterial meningitis; adventitia; cerebral arteritis; internal elastic lamina; intima; media.
Copyright: © 2025 Brain Circulation.
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- Loewenstein Ueber die veränderungen des gehirns und rückenmarks bei meningitis cerebrospinalis. Beitr Path Anat Allg Path. 1910;47:282.
-
- Ernst P. Das nervensystem. In: Aschoff L, editor. Pathologische Anatomie. Jena: Gustav Fischer; 1928.
-
- MacCallum WG. Philadelphia: W. B. Saunders Company; 1926. Textbook of Pathology.
-
- Wertham F. The cerebral lesions in purulent meningitis. Arch Neurol Psychiatry. 1931;26:549.
-
- Buzzard EF, Greenfield JG. London: Constable and Company, Ltd; 1921. Pathology of the Nervous System.
Publication types
LinkOut - more resources
Full Text Sources
