The matricellular protein ADAMTS-like 2 regulates differentiation of skeletal muscle-resident fibro-adipogenic progenitor cells
- PMID: 40520105
- PMCID: PMC12164049
- DOI: 10.1016/j.isci.2025.112712
The matricellular protein ADAMTS-like 2 regulates differentiation of skeletal muscle-resident fibro-adipogenic progenitor cells
Abstract
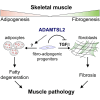
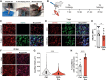
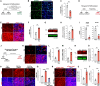
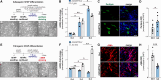
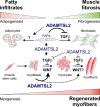
Skeletal muscle development and regeneration requires the activities of myogenic and non-myogenic muscle stem cell populations. Non-myogenic muscle stem cells, such as fibro-adipogenic progenitors (FAPs), play important roles in muscle regeneration after injury. Activated FAPs promote myogenic muscle stem cell differentiation and contribute to the restoration of muscle architecture. In pathological conditions, FAPs can differentiate into adipocytes or fibroblasts, causing fatty infiltrations or muscle fibrosis, respectively. Here, we identified the extracellular matrix protein ADAMTS-like 2 (ADAMTSL2) as a regulator of adipogenic and fibrogenic FAP differentiation. In the context of fibrogenic FAP differentiation, ADAMTSL2 inhibited the differentiation of primary mouse and human FAPs into fibroblasts in a transforming growth factor β (TGF-β)-dependent manner. Together with our previous data, a model emerges where ADAMTSL2 has a dual role in skeletal muscle biology, a pro-myogenic role, where ADAMTSL2 promotes myogenic muscle stem cell differentiation, and a TGF-β-dependent anti-fibrotic role where ADAMTSL2 attenuates FAP-to-fibroblast differentiation.
Keywords: Biochemistry; Cell biology; Specialized functions of cells.
© 2025 The Author(s).
Conflict of interest statement
J.H.C. and K.J.H. are employees of Cook MyoSite Inc.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

