Spatiotemporal properties of cortical excitatory and inhibitory neuron activation by sustained and bursting electrical microstimulation
- PMID: 40520112
- PMCID: PMC12167498
- DOI: 10.1016/j.isci.2025.112707
Spatiotemporal properties of cortical excitatory and inhibitory neuron activation by sustained and bursting electrical microstimulation
Abstract
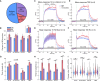
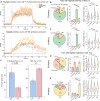
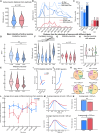
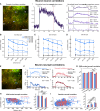
Intracortical microstimulation (ICMS) of sensory brain regions can create artificial sensations, yet these percepts fade with continued stimulation, suggesting dynamic changes in underlying neural activity. Using two-photon imaging in transgenic mice, this study examines how prolonged ICMS (30 s) shapes activity in excitatory and inhibitory neurons within the visual cortex. Inhibitory neuron activity was more likely to increase throughout the stimulation period, while excitatory neuron activity was more likely to decrease and be suppressed post-stimulation. Stimulation patterns differentially shaped neuronal engagement: theta-burst stimulation most effectively activated inhibitory neurons, whereas 10-Hz burst most effectively activated excitatory neurons. ICMS evoked more diverse responses in neurons farther from the electrode, reflecting complex synaptic interactions involving inhibition and disinhibition. These results show that ICMS affects excitatory and inhibitory neurons differently over extended durations. Modulation of stimulation patterns may allow for preferential engagement of specific neuron types and shaping of cortical activity.
Keywords: Neuroscience; Sensory neuroscience.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Neural mechanisms of the temporal response of cortical neurons to intracortical microstimulation.Brain Stimul. 2024 Mar-Apr;17(2):365-381. doi: 10.1016/j.brs.2024.03.012. Epub 2024 Mar 16. Brain Stimul. 2024. PMID: 38492885 Free PMC article.
-
Dynamic amplitude modulation of microstimulation evokes biomimetic onset and offset transients and reduces depression of evoked calcium responses in sensory cortices.Brain Stimul. 2023 May-Jun;16(3):939-965. doi: 10.1016/j.brs.2023.05.013. Epub 2023 May 25. Brain Stimul. 2023. PMID: 37244370 Free PMC article.
-
Intracortical microstimulation pulse waveform and frequency recruits distinct spatiotemporal patterns of cortical neuron and neuropil activation.J Neural Eng. 2022 Mar 31;19(2):10.1088/1741-2552/ac5bf5. doi: 10.1088/1741-2552/ac5bf5. J Neural Eng. 2022. PMID: 35263736 Free PMC article.
-
The temporal pattern of intracortical microstimulation pulses elicits distinct temporal and spatial recruitment of cortical neuropil and neurons.J Neural Eng. 2021 Jan 25;18(1):10.1088/1741-2552/abc29c. doi: 10.1088/1741-2552/abc29c. J Neural Eng. 2021. PMID: 33075762 Free PMC article.
-
Neural hijacking: action of high-frequency electrical stimulation on cortical circuits.Neuroscientist. 2013 Oct;19(5):434-41. doi: 10.1177/1073858412458368. Epub 2012 Sep 10. Neuroscientist. 2013. PMID: 22968640 Free PMC article. Review.
References
-
- Flesher S.N., Collinger J.L., Foldes S.T., Weiss J.M., Downey J.E., Tyler-Kabara E.C., Bensmaia S.J., Schwartz A.B., Boninger M.L., Gaunt R.A. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 2016;8:361ra141. - PubMed
LinkOut - more resources
Full Text Sources

