Biochemical characterization of Bifidobacterium bifidum peptidoglycan d,l-endopeptidase BbMep that generates NOD2 ligands
- PMID: 40520142
- PMCID: PMC12160582
- DOI: 10.1039/d5cb00086f
Biochemical characterization of Bifidobacterium bifidum peptidoglycan d,l-endopeptidase BbMep that generates NOD2 ligands
Abstract
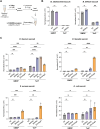
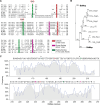
Soluble peptidoglycan fragments produced by the gut bacteria are key effectors in microbiota-host crosstalk. Here, we biochemically characterized BbMep, an NlpC/p60 domain-containing peptidoglycan d,l-endopeptidase from Bifidobacterium bifidum, which efficiently digests Lys- or Orn-type sacculi. Digestion of human stool-derived muropeptides by BbMep enhances NOD2 activation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Enterococcus faecium secreted antigen A generates muropeptides to enhance host immunity and limit bacterial pathogenesis.Elife. 2019 Apr 10;8:e45343. doi: 10.7554/eLife.45343. Elife. 2019. PMID: 30969170 Free PMC article.
-
2'-Fucosyllactose synbiotics with Bifidobacterium bifidum to improve intestinal transcriptional function and gut microbiota in constipated mice.Food Res Int. 2025 Oct;217:116840. doi: 10.1016/j.foodres.2025.116840. Epub 2025 Jun 9. Food Res Int. 2025. PMID: 40597542
-
Bifidobacterium bifidum G9-1 Survives in the Intestinal Environment and Influences the Gut Microbiota Despite the Presence of Antimicrobials.Microbiol Immunol. 2025 Jun 29. doi: 10.1111/1348-0421.13230. Online ahead of print. Microbiol Immunol. 2025. PMID: 40582892
-
Effect of Fructooligosaccharides Supplementation on the Gut Microbiota in Human: A Systematic Review and Meta-Analysis.Nutrients. 2022 Aug 12;14(16):3298. doi: 10.3390/nu14163298. Nutrients. 2022. PMID: 36014803 Free PMC article.
-
Gut bacterial profiles in Parkinson's disease: A systematic review.CNS Neurosci Ther. 2023 Jan;29(1):140-157. doi: 10.1111/cns.13990. Epub 2022 Oct 25. CNS Neurosci Ther. 2023. PMID: 36284437 Free PMC article.
References
-
- Schwarzer M. Gautam U. K. Makki K. Lambert A. Brabec T. Joly A. Šrůtková D. Poinsot P. Novotná T. Geoffroy S. Courtin P. Hermanová P. P. Matos R. C. Landry J. J. M. Gérard C. Bulteau A.-L. Hudcovic T. Kozáková H. Filipp D. Chapot-Chartier M.-P. Šinkora M. Peretti N. Boneca I. G. Chamaillard M. Vidal H. De Vadder F. Leulier F. Science. 2023;379:826–833. doi: 10.1126/science.ade9767. - DOI - PubMed
LinkOut - more resources
Full Text Sources

