A systematic review and meta-analysis of efruxifermin's efficacy in improving liver fibrosis in patients with NASH/MASH
- PMID: 40520164
- PMCID: PMC12163238
- DOI: 10.3389/fphar.2025.1594091
A systematic review and meta-analysis of efruxifermin's efficacy in improving liver fibrosis in patients with NASH/MASH
Abstract
Background & aims: Efruxifermin is a potential treatment for non-alcoholic steatohepatitis (NASH, now termed metabolic dysfunction-associated steatohepatitis, MASH). This study aimed to analyze the effectiveness of efruxifermin in improving liver fibrosis in patients with MASH.
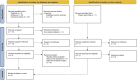
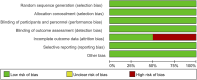
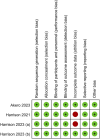
Methods: Systematic searches of PubMed, Cochrane Library, and Embase databases were conducted. Randomized controlled trials evaluating the efficacy of efruxifermin compared with placebo in patients with MASH were included. The primary outcome was the proportion of patients with improvement in liver fibrosis by 1 or more stages without worsening of MASH. The secondary outcomes were non-invasive biomarkers of fibrosis and treatment-emergent adverse events (TEAEs).
Results: This meta-analysis included 4 studies with a total of 325 patients with biopsy-proven MASH and stage F1-F4 fibrosis. All studies reported histological outcomes. Compared to placebo, efruxifermin demonstrated a higher relative risk (RR) of 1.97 (95% CI 1.21 to 3.19, I 2 = 0%, P = 0.006) for achieving improvement in fibrosis by ≥ 1 stage without worsening of MASH. Furthermore, efruxifermin improved fibrosis-related non-invasive biomarkers (enhanced liver fibrosis [ELF]score, N-terminal type-III collagen pro-peptide [ProC3], and liver stiffness by FibroScan). However, efruxifermin was associated with an increased risk of adverse events compared to placebo, but this finding was not robust in sensitivity analysis.
Conclusion: Efruxifermin may be a potential therapeutic for MASH-related fibrosis, with the available data indicating seemingly favorable tolerability.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023491895, CRD42023491895.
Keywords: FGF21; MASH; efruxifermin; fibrosis; non-alcoholic; steatohepatitis.
Copyright © 2025 Li, Zou, Zhou, Li, Liao, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
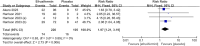
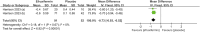
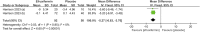

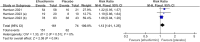
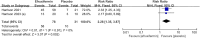
References
-
- Akero (2023). Phase 2b SYMMETRY readout.
-
- Brunt E. M., Kleiner D. E., Wilson L. A., Sanyal A. J., Neuschwander-Tetri B. A. Nonalcoholic Steatohepatitis Clinical Research Network (2019). Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: results from the nonalcoholic steatohepatitis clinical research network treatment trials. Hepatology 70 (2), 522–531. 10.1002/hep.30418 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

