Biodegradable sustained-release microneedle patch loaded with clindamycin hydrochloride: a breakthrough in acne management
- PMID: 40520168
- PMCID: PMC12162646
- DOI: 10.3389/fphar.2025.1575635
Biodegradable sustained-release microneedle patch loaded with clindamycin hydrochloride: a breakthrough in acne management
Abstract
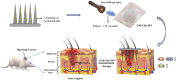
Background: Clindamycin hydrochloride, a first-line antibiotic for acne treatment, faces challenges with poor skin penetration due to its hydrophilicity and the barrier posed by the stratum corneum. To address this limitation, we developed gelatin-methacryloyl (GelMA) hydrogel-based biodegradable microneedles (GM-Clin-MN) for sustained intradermal drug delivery, thereby enhancing therapeutic efficacy.
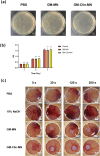
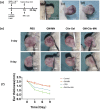
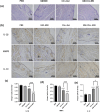
Methods: The microneedle patches loaded with 1 wt% clindamycin hydrochloride were fabricated using PDMS molds and characterized through scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and fluorescence microscopy. Drug loading and release were assessed using UV-Vis spectroscopy at 520 nm, while mechanical strength was evaluated with a universal testing machine. Skin penetration was tested on ex vivo rat abdominal skin. Biosafety was determined through human skin fibroblast (HSF) cytotoxicity and hen's egg test-chorioallantoic membrane (HET-CAM) irritation tests. Antibacterial efficacy against Cutibacterium acnes (C. acnes) was measured via colony counting. In vivo acne treatment of the microneedles was evaluated in a rat acne model. Gross morphological changes, histological sections, and immunohistochemical staining were used to evaluate the efficacy and potential mechanisms of acne treatment.
Results: Clindamycin hydrochloride-loaded GelMA microneedles (GM-Clin-MN) achieved a drug loading of 0.49 ± 0.025 μg/needle, exhibiting rapid release on Day 1 (54.8% ± 2.1%) and sustained release by Day 10 (72.1% ± 1.5%). The microneedles penetrated the skin to a depth of 658 ± 66 μm, swelled by 185.4% ± 12.1%, and completely dissolved within 10 min. GM-Clin-MN displayed no cytotoxicity or skin irritation and effectively inhibited the growth of C. acnes (bacterial inhibition rate of 100%). In vivo studies revealed that acne-related inflammation was effectively suppressed with potential anti-scarring properties, characterized by reduced pro-inflammatory IL-1β levels, increased anti-inflammatory IL-10 expression, and diminished MMP-2 activity - a key enzyme in collagen overproduction during scarring.
Conclusion: GM-Clin-MN enables sustained, minimally invasive clindamycin delivery through the stratum corneum, offering a dual-action therapeutic strategy that combines potent antibacterial activity with anti-inflammatory modulation for acne management.
Keywords: GelMA hydrogel; acne vulgaris; clindamycin hydrochloride; minimally invasive drug delivery system; swellable microneedles.
Copyright © 2025 Fan, Liao, Yang, Xing, Zhang, Luo, Pu, Wu, Li, Zhao and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
[Preparation of a Microneedle Patch Loaded With Sodium Houttuyfonate Combined With Erythromycin and Its Antiacne Effects].Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Jan 20;56(1):268-276. doi: 10.12182/20250160206. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40109482 Free PMC article. Chinese.
-
Pre-assembled nanospheres in mucoadhesive microneedle patch for sustained release of triamcinolone in the treatment of oral submucous fibrosis.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Aug 28;49(8):1245-1260. doi: 10.11817/j.issn.1672-7347.2024.240226. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39788513 Free PMC article.
-
Development and Characterization of a Hydrogel Containing Chloramphenicol-Loaded Binary Ethosomes for Effective Transdermal Permeation and Treatment Acne in Rat Model.Int J Nanomedicine. 2025 Feb 6;20:1697-1715. doi: 10.2147/IJN.S476937. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39931531 Free PMC article.
-
Clindamycin phosphate 1.2% / tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris.J Eur Acad Dermatol Venereol. 2015 Jun;29 Suppl 5:8-13. doi: 10.1111/jdv.13185. J Eur Acad Dermatol Venereol. 2015. PMID: 26059820 Review.
-
Clindamycin/benzoyl peroxide gel: a review of its use in the management of acne.Am J Clin Dermatol. 2002;3(5):349-60. doi: 10.2165/00128071-200203050-00007. Am J Clin Dermatol. 2002. PMID: 12069641 Review.
References
-
- Ali A., Khatoon S., Alam M., Bhat S. (2024). Unani perspective of buthūr labaniyya (acne vulgaris): a comprehensive review. Int. J. Pharmacogn. Life Sci. 5 (1), 124–132. 10.33545/27072827.2024.v5.i1b.117 - DOI
-
- Bhavsar B., Choksi B., Sanmukhani J., Dogra A., Haq R., Mehta S., et al. (2014). Clindamycin 1% nano-emulsion gel formulation for the treatment of acne vulgaris: results of a randomized, active controlled, multicentre, phase IV clinical trial. J. Clin. Diagn. Res. 8, YC05–09. 10.7860/JCDR/2014/9111.4769 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

