Detecting benzodiazepine use through induced eye convergence inability with a smartphone app: a proof-of-concept study
- PMID: 40520217
- PMCID: PMC12162636
- DOI: 10.3389/fdgth.2025.1584716
Detecting benzodiazepine use through induced eye convergence inability with a smartphone app: a proof-of-concept study
Abstract
Background: Benzodiazepines (BZDs) are readily available potent drugs that act as central depressants. These drugs are widely used, misused, and abused. For patients with BZD use disorder, the traditional sobriety monitoring method is periodic urine tests.
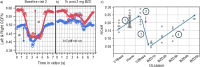
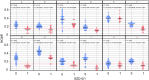
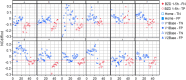
Methods: The utility of eye-scanning data related to non-convergence (the ability to cross eyes) collected using smartphones with the Previct Drugs app before and after ingestion of the BZD lorazepam for detecting BZD-driven effects was evaluated using data from 12 individuals from a historic clinical study (NCT05731999). Using a novel metric that represents the change in distance between irises when converging eyes, either in absolute terms (NCdiff) or individualized (NCdiffInd), classifiers were built using logistic regression.
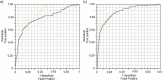
Results: The ability to converge eyes is a strongly individual and acquired skill that is impaired after ingesting lorazepam. The maximum NCdiff for a BZD-sober individual may be smaller than the impaired NCdiff for another individual. Using the NCdiff measured in a sober condition after approximately 1 week of regular eye-scanning as the individual baseline to form NCdiffInd produced a highly functional classifier with an area under the curve (AUC) = 0.88, which was superior to a classifier based on NCdiff with an AUC = 0.79.
Conclusions: The loss of eye convergence induced by lorazepam is continuous, individual, and can be partial. Smartphone-based eye-scanning technology combined with a classifier adapted to the ability of eye convergence of individuals shows promising performance in detecting ingestion of lorazepam.
Keywords: benzodiazepines; eye convergence; pupillometry; smartphone; substance use disorder.
© 2025 Kuijpers, Hämäläinen, Zetterström, Winkvist, Niesters, van Velzen, Nyberg, Dahan and Andersson.
Conflict of interest statement
MH and AZ is and MW was at the time of authoring this manuscript employees of Kontigo Care AB that have made a product partly based on the results of the presented results (Previct Drugs). KA is an employee of Skillsta Teknik Design och Kvalitet AB, which is a subcontractor to Kontigo Care AB. KA, AZ and MH are co-inventors of two (MH) and three (KA, AZ) different patents that are related to the presented work [PCT/SE2023/050638 “Quality assurance in body images”, PCT/SE2023/051070; “Method for estimating pupil size” (only KA and AZ), PCT/SE2023/051071; “Method and system for selfadministered surveillance of use of addictive stimulus”]. FN is part of the advisory board of Kontigo Care AB. The authors declare that this study received funding from Kontigo Care. The funder had the following involvement in the study: Study design, interpretation of data, and the writing of the manuscript. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
[In utero exposure to benzodiazepine. Is there a risk for anal atresia with lorazepam?].Encephale. 2003 Nov-Dec;29(6):553-9. Encephale. 2003. PMID: 15029090 Review. French.
-
Eye reactions under the influence of drugs of abuse as measured by smartphones: a controlled clinical study in healthy volunteers.Front Neurosci. 2025 Jan 7;18:1492246. doi: 10.3389/fnins.2024.1492246. eCollection 2024. Front Neurosci. 2025. PMID: 39840007 Free PMC article.
-
Urine benzodiazepine screening using Roche Online KIMS immunoassay with beta-glucuronidase hydrolysis and confirmation by gas chromatography- mass spectrometry.J Anal Toxicol. 2005 Apr;29(3):193-200. doi: 10.1093/jat/29.3.193. J Anal Toxicol. 2005. PMID: 15842763
-
Validation of a Smartphone Pupillometry Application in Diagnosing Severe Traumatic Brain Injury.J Neurotrauma. 2023 Oct;40(19-20):2118-2125. doi: 10.1089/neu.2022.0516. Epub 2023 Aug 16. J Neurotrauma. 2023. PMID: 37464770
-
Beyond benzodiazepines: a meta-analysis and narrative synthesis of the efficacy and safety of alternative options for alcohol withdrawal syndrome management.Eur J Clin Pharmacol. 2023 Sep;79(9):1147-1157. doi: 10.1007/s00228-023-03523-2. Epub 2023 Jun 28. Eur J Clin Pharmacol. 2023. PMID: 37380897 Review.
References
-
- Griffin CE, 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. (2013) 13(2):214–23. Available at: https://www.ochsnerjournal.org/content/13/2/214 - PMC - PubMed
-
- Robertson S, Peacock EE, Scott R. Benzodiazepine use disorder: common questions and answers. Am Fam Physician. (2023) 108(3):260–6. Available at: https://www.aafp.org/pubs/afp/issues/2023/0900/benzodiazepine-use-disord... - PubMed
LinkOut - more resources
Full Text Sources

