Hepatic Manifestations Following Gene Therapy
- PMID: 40520419
- PMCID: PMC12166697
- DOI: 10.1016/j.gastha.2025.100681
Hepatic Manifestations Following Gene Therapy
Abstract
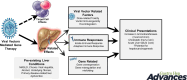
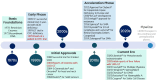
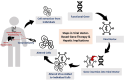
Gene therapy involves the introduction of genetic materials, most commonly using viral vectors, to alter gene expression to ameliorate or cure disease symptoms with minimal adverse events. While interest in gene therapy has been on the increase, concerns have arisen about the potential for hepatotoxicity, which arises from diverse mechanisms. While viral vectors can produce dose-dependent hepatotoxicity secondary to integration, immune responses appear to be the primary driving mechanism. A mild increase in aminotransferases is the most common hepatic manifestation, occurring variably in 20%-80%, while there has been rare progress to acute liver failure. The occurrence of hepatotoxicity is unpredictable and can vary depending on patient comorbidities, vector dose, vector type, and degree of immune activation. Pretreatment screening for underlying chronic liver disease and exclusion of advanced fibrosis or cirrhosis using noninvasive tests is essential. Literature on liver biopsy pre- and post-therapy is limited, but small studies show safety in the long term. Immunosuppression, most commonly using corticosteroids, is the first-line modality in management. Approaches vary between prophylactic and reactive strategies, and there remains an absence of consensus on the most appropriate strategies. First-line therapy for a variable duration and dose settles most cases of hepatotoxicity. In selected difficult-to-treat cases, second-line agents (sirolimus, mycophenolate mofetil, and calcineurin inhibitors) are required, while there is no current consensus on the ideal second-line strategy. Intense short-term and extended long-term hepatic monitoring is recommended. Variabilities in presentation and challenges in management strategies mandate a multidisciplinary collaboration with the active involvement of hepatologists/gastroenterologists to optimize liver health.
Keywords: Adenovirus; Corticosteroids; Gene vectors; Hepatotoxicity; Liver injury; Transgene.
© 2025 The Authors.
Figures

Similar articles
-
[Chinese experts consensus statement: diagnosis and treatment of cystic fibrosis (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2023 Apr 12;46(4):352-372. doi: 10.3760/cma.j.cn112147-20221214-00971. Zhonghua Jie He He Hu Xi Za Zhi. 2023. PMID: 36990700 Chinese.
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Vesicoureteral Reflux.2024 Apr 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Apr 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 33085409 Free Books & Documents.
-
[Methotrexate, liver and rheumatoid arthritis in tropical areas].Sante. 2001 Jul-Sep;11(3):195-200. Sante. 2001. PMID: 11641084 Review. French.
-
Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option?Paediatr Drugs. 2011 Feb 1;13(1):49-69. doi: 10.2165/11538530-000000000-00000. Paediatr Drugs. 2011. PMID: 21162600 Review.
References
-
- High K.A., Roncarolo M.G. Gene therapy. New Engl J Med. 2019;381:455–464. - PubMed
-
- Sayed N., Allawadhi P., Khurana A., et al. Gene therapy: comprehensive overview and therapeutic applications. Life Sci. 2022;294 - PubMed
-
- Whiteley L.O. An overview of Nonclinical and clinical liver toxicity associated with AAV gene therapy. Toxicologic Pathol. 2023;51:400–404. - PubMed
-
- Zabaleta N., Unzu C., Weber N.D., et al. Gene therapy for liver diseases — progress and challenges. Nat Rev Gastroenterol Hepatol. 2023;20:288–305. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

