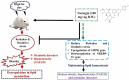
Naringin ameliorates high-fat diet-induced hepatotoxicity and dyslipidemia in experimental rat model via modulation of anti-oxidant enzymes, AMPK and SERBP-1c signaling pathways
- PMID: 40520525
- PMCID: PMC12167040
- DOI: 10.1016/j.toxrep.2025.102062
Naringin ameliorates high-fat diet-induced hepatotoxicity and dyslipidemia in experimental rat model via modulation of anti-oxidant enzymes, AMPK and SERBP-1c signaling pathways
Abstract
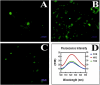
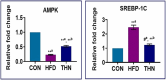
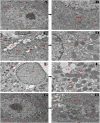
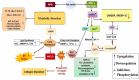
High-fat diet causes elevation of steatosis, dyslipidemia and oxidative stress which eventually leads to hepatic injury in the form of non-alcoholic fatty liver disease (NAFLD). Naringin, a natural flavonoid, having tremendous potentiality including antioxidant, anti-inflammatory, hypolipidemic role. Based on this proposition, we investigated the role of naringin in hepatotoxicity and its possible underlying mechanism caused by high-fat diet for prolonged time. Fifteen Wistar rats were divided into three groups: Group A (CON) received normal diet; Group B (HFD) was administered with high-fat diet for 16 weeks; and Group C (THN) was treated with naringin (100 mg/kg B.W.) for last 6 weeks after induction of obesity. After autopsy, various parameters were studied like gravimetry, serum biochemistry, ROS activity, anti-oxidant enzymes, genes expression (AMPK and SREBP-1C), histochemistry, histopathology and ultrastructure of hepatic tissue. In HFD group, Masson's trichome stain intensity increased 6.8-folds, indicating the onset of liver fibrosis; ROS generation and lipid peroxidation (TBARS) were significantly (p < 0.01) increased, whereas SOD and CAT were decreased by 36.7 % and 49.7 %, respectively. Furthermore, these parameters were remained normal in THN group. Besides, HFD group displayed extreme elevation in hepatic SREBP-1C expression (147 %) and downregulation of AMPK gene (77 %) compared to control. The ultrastructural study revealed most important and new insight of this study where HFD induced extreme reticule stress in hepatic tissue which was significantly improved by the treatment of naringin. These findings demonstrate that the naringin may be used as a potential therapeutic agent to combat obesity related hyperlipidemia and NAFLD.
Keywords: AMPK signaling; HFD; Hepatotoxicity; Lipid metabolism; NAFLD; Naringin; Oxidative stress.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
Potential Synergistic Effects of Caffeine and Naringin on Mitochondrial Biogenesis and Hepatic Steatosis in Adult Male Rats With NAFLD Induced by a High-Fat Diet.Biomed Res Int. 2025 Aug 13;2025:1565994. doi: 10.1155/bmri/1565994. eCollection 2025. Biomed Res Int. 2025. PMID: 40842941 Free PMC article.
-
[Effect of Wenpi Pills on lipid metabolism in mice with non-alcoholic fatty liver disease induced by various diets].Zhongguo Zhong Yao Za Zhi. 2025 May;50(10):2730-2739. doi: 10.19540/j.cnki.cjcmm.20250120.401. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40686142 Chinese.
-
Synergies of dibutyl phthalate on high-fat diet can aggravate cardiac fibrosis/dysfunction and the protective effects of vitamin E and salidroside: A molecular toxicological study in Sprague-Dawley rats.Ecotoxicol Environ Saf. 2025 Sep 1;302:118708. doi: 10.1016/j.ecoenv.2025.118708. Epub 2025 Jul 19. Ecotoxicol Environ Saf. 2025. PMID: 40684633
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.Cochrane Database Syst Rev. 2013 Dec 27;2013(12):CD008623. doi: 10.1002/14651858.CD008623.pub2. Cochrane Database Syst Rev. 2013. PMID: 24374462 Free PMC article.
References
-
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. - DOI - PMC - PubMed
-
- WHO, Obesity and overweight. 2024. World Health Organization, Switzerland, (n.d.).
LinkOut - more resources
Full Text Sources
Miscellaneous

