Breaking the psoriasis pathological signaling cycle: A novel nanomedicine strategy targeting metabolism and oxidative stress
- PMID: 40520564
- PMCID: PMC12164231
- DOI: 10.1016/j.mtbio.2025.101887
Breaking the psoriasis pathological signaling cycle: A novel nanomedicine strategy targeting metabolism and oxidative stress
Abstract
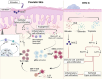
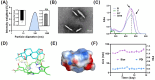
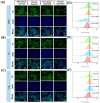
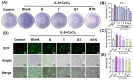
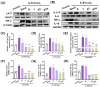
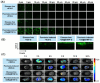
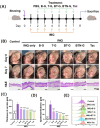
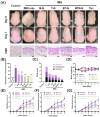
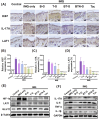
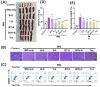
Psoriasis is a chronic skin disorder characterized by dysregulation of immune and epithelial cells, resulting in persistent symptoms such as erythema, scaling, and induration. The abnormal metabolism and increased oxidative stress in psoriasis lesions have been identified as key drivers in the pathogenesis of psoriasis, forming a positive feedback loop within psoriatic skin. Therefore, targeting this feedback loop through modulation of local metabolism and alleviation of oxidative stress could be a rational and promising therapeutic strategy for addressing psoriasis. Herein, we designed a carrier-free nanomedicine (BTN) incorporating bilirubin (BR) and triptolide (TPL) to specifically target two key pathological features of psoriasis: inflammation induced by enhanced reactive oxygen species (ROS) and aberrant proliferation/immune activation driven by heightened nutrient metabolism. In vitro studies demonstrated that BTN effectively improved the water solubility of BR and TPL while facilitating efficient drug delivery to inflammatory keratinocytes. Mechanistically, BTN was found to alleviate the inflammatory cascade caused by oxidative stress and inhibit the IL-23/IL-17 axis. Importantly, downregulation of HIF-1α in keratinocytes resulted in blocking glucose transportation via GLUT-1 as well as amino acid transportation via LAT1, ultimately impeding excessive proliferation by disrupting nutritional requirements. In an imiquimod-induced psoriasis model, BTN effectively permeated inflamed skin epithelium with long-term retention effect. As a multifunctional nanomedicine combining ROS scavenging properties with regulation of nutrition metabolism, BTN shows great promise for reducing inflammatory cell infiltration and suppressing keratinocyte proliferation. Our findings demonstrated the great potential of BTN in ameliorating psoriasis symptoms by restoring the metabolic imbalance and mitigating oxidative stress between the epithelial and immune compartments.
Keywords: Bilirubin; HIF-1α; Nanomedicine; Psoriasis; Triptolide.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Bilirubin nanomedicine alleviates psoriatic skin inflammation by reducing oxidative stress and suppressing pathogenic signaling.J Control Release. 2020 Sep 10;325:359-369. doi: 10.1016/j.jconrel.2020.07.015. Epub 2020 Jul 16. J Control Release. 2020. PMID: 32681946
-
Glutamine-Based Metabolism Normalization and Oxidative Stress Alleviation by Self-Assembled Bilirubin/V9302 Nanoparticles for Psoriasis Treatment.Adv Healthc Mater. 2023 May;12(13):e2203397. doi: 10.1002/adhm.202203397. Epub 2023 Feb 3. Adv Healthc Mater. 2023. PMID: 36690435
-
Microneedle system carrying Momordin Ic-loaded ROS-responsive hydrogel ameliorates psoriasis via targeted anti-inflammatory and reactive oxygen species (ROS)-scavenging mechanisms.Int J Pharm. 2025 May 15;676:125530. doi: 10.1016/j.ijpharm.2025.125530. Epub 2025 Apr 6. Int J Pharm. 2025. PMID: 40199433
-
Hypoxia-inducible factor-1: A potential pharmacological target to manage psoriasis.Int Immunopharmacol. 2020 Sep;86:106689. doi: 10.1016/j.intimp.2020.106689. Epub 2020 Jun 22. Int Immunopharmacol. 2020. PMID: 32585606 Review.
-
Unraveling Mitochondrial Reactive Oxygen Species Involvement in Psoriasis: The Promise of Antioxidant Therapies.Antioxidants (Basel). 2024 Oct 11;13(10):1222. doi: 10.3390/antiox13101222. Antioxidants (Basel). 2024. PMID: 39456475 Free PMC article. Review.
References
-
- Boehncke W.H., Schon M.P. Psoriasis. Lancet. 2015;386(9997):983–994. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

