Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells via injectable microfluidic-templated microgels for retinal regeneration
- PMID: 40520565
- PMCID: PMC12163166
- DOI: 10.1016/j.mtbio.2025.101880
Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells via injectable microfluidic-templated microgels for retinal regeneration
Abstract
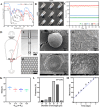
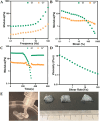
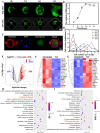
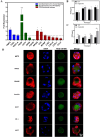
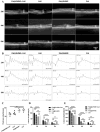
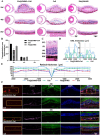
Retinal pigment epithelial (RPE) cells are specialized neural cells crucial for vision, while human embryonic stem cell-derived retinal pigment epithelial (hESC-RPE) cells hold great potential as a viable cell source for treating retinal degenerative diseases like retinitis pigmentosa (RP). However, the transplantation efficiency and viability of hESC-RPE cell suspensions are relatively low due to detrimental shear-force during operations and host immune-clearance. We herein develop an alternative transplantation strategy with the aid of a microfluidic-templating microgel cell carrier to achieve substantially enhanced loading and delivery efficiency of hESC-RPE cells, thereby promoting visual function recovery after subretinal injection in the RP model Royal College of Surgeons (RCS) rats. Specifically, injectable monodispersed microgels consisting of gelatin-methacryloyl/Hyaluronic acid-methacryloyl core coated with fibrin shell (denoted as Fib@GHMS) were fabricated in a high-throughput and controllable manner, facilitating the adhesion and proliferation of hESC-RPE cells. RCS rats treated with microcarriers showed significantly improved visual function, evidenced by increased B-wave amplitudes and the preservation of the inner nuclear layer at 8 weeks post-surgery. In conclusion, our innovative delivery system Fib@GHMS for hESC-RPE cell transplantation presents a potential therapeutic graft for retinal tissue engineering. It may open a new avenue for clinical transplantation of minimally invasive cell-based treatments in retinal degenerative diseases.
Keywords: Cell transplantation; Cell-laden microgel; Injectable granular gel; Microfluidics; hESC-RPE.
© 2025 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Hydrogels to Support Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells.Brain Sci. 2022 Nov 25;12(12):1620. doi: 10.3390/brainsci12121620. Brain Sci. 2022. PMID: 36552081 Free PMC article.
-
Improving cell survival and engraftment in vivo via layer-by-layer nanocoating of hESC-derived RPE cells.Stem Cell Res Ther. 2020 Nov 25;11(1):495. doi: 10.1186/s13287-020-01986-z. Stem Cell Res Ther. 2020. PMID: 33239074 Free PMC article.
-
Functional assessment of cryopreserved clinical grade hESC-RPE cells as a qualified cell source for stem cell therapy of retinal degenerative diseases.Exp Eye Res. 2021 Jan;202:108305. doi: 10.1016/j.exer.2020.108305. Epub 2020 Oct 17. Exp Eye Res. 2021. PMID: 33080300
-
[Retinal pigment epithelial cell transplantation: perspective].Nippon Ganka Gakkai Zasshi. 1996 Dec;100(12):982-1006. Nippon Ganka Gakkai Zasshi. 1996. PMID: 9022310 Review. Japanese.
-
A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models.Int J Mol Sci. 2020 Apr 14;21(8):2719. doi: 10.3390/ijms21082719. Int J Mol Sci. 2020. PMID: 32295315 Free PMC article.
References
LinkOut - more resources
Full Text Sources

