Molecular detection of Leishmania and other vector-borne agents in free-ranging and captive herpetofauna from Costa Rica
- PMID: 40520770
- PMCID: PMC12167032
- DOI: 10.1016/j.ijppaw.2025.101090
Molecular detection of Leishmania and other vector-borne agents in free-ranging and captive herpetofauna from Costa Rica
Abstract
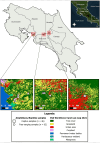
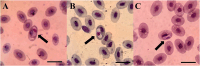
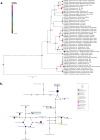
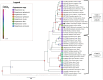
Vector-borne pathogens in amphibians and reptiles represent an emerging concern in wildlife, with implications for ecosystem dynamics and potential zoonotic risks. In this study, we screened 108 animals from Costa Rica, including 46 captive snakes, 24 free-ranging reptiles, and 38 free-ranging amphibians, for the presence of Trypanosomatidae, Anaplasmataceae, Borrelia, Rickettsia, and Hepatozoon spp. Blood smear analysis revealed protozoa gametocytes in 3.7 % of the animals sampled, and 11.1 % of amphibians and reptiles were molecular positive for at least one pathogen. Specifically, 7.4 % of the samples tested positive for Leishmania spp., 1.85 % for Trypanosoma spp., 0.9 % for Anaplasma spp., and 1.85 % for Hepatozoon spp. Notably, this study reports the first molecular detection of Leishmania in an amphibian species (Rhinella horribilis) and confirms the presence of mammalian pathogenic Leishmania infantum in captive snakes in Central America. The presence of potential zoonotic agents in both captive and free-ranging herpetofauna underscores the importance of screening wildlife species, including understudied host groups such as amphibians, to better understand their role in disease ecology.
Keywords: Amphibians; Leishmania infantum; Protozoa; Reptiles; Wildlife.
© 2025 The Authors. Published by Elsevier Ltd on behalf of Australian Society for Parasitology.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Molecular detection of vector-borne agents in ectoparasites and reptiles from Brazil.Ticks Tick Borne Dis. 2021 Jan;12(1):101585. doi: 10.1016/j.ttbdis.2020.101585. Epub 2020 Oct 6. Ticks Tick Borne Dis. 2021. PMID: 33113476
-
Seroprevalence and Current Infections of Canine Vector-Borne Diseases in Costa Rica.Front Vet Sci. 2019 Jun 4;6:164. doi: 10.3389/fvets.2019.00164. eCollection 2019. Front Vet Sci. 2019. PMID: 31214605 Free PMC article.
-
Molecular Survey on Vector-Borne Pathogens in Alpine Wild Carnivorans.Front Vet Sci. 2020 Jan 23;7:1. doi: 10.3389/fvets.2020.00001. eCollection 2020. Front Vet Sci. 2020. PMID: 32039255 Free PMC article.
-
Reptile vector-borne diseases of zoonotic concern.Int J Parasitol Parasites Wildl. 2021 Apr 22;15:132-142. doi: 10.1016/j.ijppaw.2021.04.007. eCollection 2021 Aug. Int J Parasitol Parasites Wildl. 2021. PMID: 34026483 Free PMC article. Review.
-
Arthropod-Borne Pathogens in Wild Canids.Vet Sci. 2023 Feb 19;10(2):165. doi: 10.3390/vetsci10020165. Vet Sci. 2023. PMID: 36851469 Free PMC article. Review.
References
-
- Attias M., Sato L.H., Ferreira R.C., Takata C.S., Campaner M., Camargo E.P., De Souza W. Developmental and ultrastructural characterization and phylogenetic analysis of Trypanosoma herthameyeri n. sp. of Brazilian Leptodactilydae frogs. J. Eukaryot. Microbiol. 2016;63:610–622. doi: 10.1111/jeu.12310. - DOI - PubMed
-
- Boggild A.K., Caumes E., Grobusch M.P., Schwartz E., Hynes N.A., Libman M., Connor B.A., Chakrabarti S., Parola P., Keystone J.S., Nash T., Showler A.J., Schunk M., Asgeirsson H., Hamer D.H., Kain C.C., GeoSentinel Surveillance Network Cutaneous and mucocutaneous leishmaniasis in travellers and migrants: a 20-year GeoSentinel Surveillance Network analysis. J. Trav. Med. 2019;26 doi: 10.1093/jtm/taz055. - DOI - PMC - PubMed
-
- Bouckaert R., Vaughan T.G., Barido-Sottani J., Duchêne S., Fourment M., Gavryushkina A., Heled J., Jones G., Kühnert D., De Maio N., Matschiner M., Mendes F.K., Müller N.F., Ogilvie H.A., du Plessis L., Popinga A., Rambaut A., Rasmussen D., Siveroni I., Suchard M.A., Wu C.H., Xie D., Zhang C., Stadler T., Drummond A.J. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019;15(4) doi: 10.1371/journal.pcbi.1006650. - DOI - PMC - PubMed
-
- Bower D.S., Brannelly L.A., McDonald C.A., Webb R.J., Greenspan S.E., Vickers M., Greenlees M.J. A review of the role of parasites in the ecology of reptiles and amphibians. Austral Ecol. 2019;44:433–448. doi: 10.1111/aec.12695. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

