Gut microbiota is associated with the disease characteristics of patients with newly diagnosed diffuse large B-cell lymphoma
- PMID: 40520871
- PMCID: PMC12163443
- DOI: 10.62347/DIMG6893
Gut microbiota is associated with the disease characteristics of patients with newly diagnosed diffuse large B-cell lymphoma
Abstract
Objective: To investigate the relationship between the gut microbiota and the biological characteristics of patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL).
Methods: This prospective study included 71 patients with newly diagnosed DLBCL. The microbiota was analyzed using 16S ribosomal DNA sequencing of fecal samples. Blood cytokines, PD-1, and PD-L1 were measured by enzyme-linked immunosorbent assay. Stratified analyses based on clinical characteristics were conducted to investigate correlations between alterations in gut microbiota and key clinicopathological parameters of DLBCL.
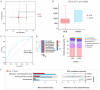
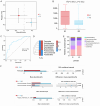
Results: In the analysis of gut microbiota heterogeneity (α diversity index), species abundance was significantly higher in the International Prognostic Index (IPI) < 3 group compared to the IPI ≥ 3 group (high-risk group). Ruminococcus was increased in the IPI ≥ 3 group. Parabacteroides, Ruminococcus, and Eubacterium were increased in the non-germinal center B-cell-like group, while Lachnospira was decreased. Megamonas was significantly increased in the high β2-microglobulin group, while Lactobacillus reuteri and Lachnospira were decreased. In the low CD4+/CD8+ ratio group, Parabacteroides was increased, while Akkermansia was decreased. Patients who failed to achieve complete remission at interim evaluation showed marked increases in Ruminococcus and Alistipes. Extranodal involvement was associated with elevated Alistipes and Enterococcus.
Conclusions: This study identified a potential relationship between the gut microbiota and DLBCL characteristics, highlighting specific gut microbiota organisms that may influence disease development and progression.
Keywords: 16S ribosomal DNA; Akkermansia; Gut microbiota; Megamonas; Parabacteroides; diffuse large B-cell lymphoma.
AJCR Copyright © 2025.
Conflict of interest statement
None.
Figures





References
-
- Shi Y, Han Y, Yang J, Liu P, He X, Zhang C, Zhou S, Zhou L, Qin Y, Song Y, Liu Y, Wang S, Jin J, Gui L, Sun Y. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res. 2019;31:152–161. - PMC - PubMed
-
- Frontzek F, Lenz G. Novel insights into the pathogenesis of molecular subtypes of diffuse large B-cell lymphoma and their clinical implications. Expert Rev Clin Pharmacol. 2019;12:1059–1067. - PubMed
-
- Jelicic J, Juul-Jensen K, Bukumiric Z, Runason Simonsen M, Kragh Jørgensen RR, Roost Clausen M, Ludvigsen Al-Mashhadi A, Schou Pedersen R, Bjørn Poulsen C, Ortved Gang A, Brown P, El-Galaly TC, Stauffer Larsen T. Validation of prognostic models in elderly patients with diffuse large B-cell lymphoma in a real-world nationwide population-based study - development of a clinical nomogram. Ann Hematol. 2025;104:433–444. - PubMed
-
- Song JY, Perry AM, Herrera AF, Chen L, Skrabek P, Nasr MR, Ottesen RA, Nikowitz J, Bedell V, Murata-Collins J, Li Y, McCarthy C, Pillai R, Wang J, Wu X, Zain J, Popplewell L, Kwak LW, Nademanee AP, Niland JC, Scott DW, Gong Q, Chan WC, Weisenburger DD. Double-hit signature with TP53 abnormalities predicts poor survival in patients with germinal center type diffuse large B-cell lymphoma treated with R-CHOP. Clin Cancer Res. 2021;27:1671–1680. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
