ZnO nanoparticles induce acute arrhythmia and heart failure in mice by disturbing cardiac ion channels
- PMID: 40520930
- PMCID: PMC12162645
- DOI: 10.3389/fcvm.2025.1569265
ZnO nanoparticles induce acute arrhythmia and heart failure in mice by disturbing cardiac ion channels
Abstract
Background: The widespread use of zinc oxide nanoparticles (ZnO NPs) has raised safety concerns on human health. However, the effects and underlying mechanisms of ZnO NPs exposure on the heart, especially during acute exposure, have yet to be elucidated.
Methods: Two different sizes of ZnO NPs (40 nm and 100 nm) were selected and their in vivo effects on mouse heart were evaluated by echocardiography and electrocardiograms. Action potential, ion channel currents, and calcium recordings were employed to assess the electrical alterations in individual myocytes. The underlying mechanisms were further investigated by transmission electron microscopy (TEM) imaging, mitochondrial staining, LDH and ROS detection. In addition, human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) were utilized for translational exploration.
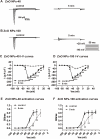
Results: Acute exposure to ZnO NPs induces cardiac dysfunction and arrhythmia in mice. Mechanistically, exposure to ZnO NPs did not significantly affect the IK1, but it markedly decreased INa and ICa-L currents, resulting in a reduced amplitude and shortened duration of the action potential in cardiomyocytes. These changes not only prolonged PR-interval and blocked A-V conduction that triggered cardiac arrhythmia, but also led to a diminished calcium transient, which contributed to heart failure. The downregulation of calcium transient upon ZnO NPs exposure was further confirmed in hiPSC-CMs. Meanwhile, acute exposure to ZnO NPs did not induce endocytosis, impair membrane integrity, or promote ROS production in the mitochondria of cardiomyocytes.
Conclusion: Acute ZnO NPs exposure causes heart failure and arrhythmia in mice by directly impacting ion channel function.
Keywords: calcium homeostasis; cardiac arrhythmia; heart failure; ion channels; zinc oxide nanoparticles.
© 2025 Liu, Deng, Zhao, Wen, Ma, Wang, Du, Li, Li, Zhang, Zhou, Soong, Yuan, Feng and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Zinc Oxide Nanoparticles Induce Mitochondrial Biogenesis Impairment and Cardiac Dysfunction in Human iPSC-Derived Cardiomyocytes.Int J Nanomedicine. 2020 Apr 21;15:2669-2683. doi: 10.2147/IJN.S249912. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32368048 Free PMC article.
-
Silica Nanoparticles Disturb Ion Channels and Transmembrane Potentials of Cardiomyocytes and Induce Lethal Arrhythmias in Mice.Int J Nanomedicine. 2020 Oct 5;15:7397-7413. doi: 10.2147/IJN.S261692. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116478 Free PMC article.
-
Food-grade titanium dioxide (E171) and zinc oxide nanoparticles induce mitochondrial permeability and cardiac damage after oral exposure in rats.Nanotoxicology. 2024 Mar;18(2):122-133. doi: 10.1080/17435390.2024.2323069. Epub 2024 Mar 4. Nanotoxicology. 2024. PMID: 38436290
-
The acute toxic effects of platinum nanoparticles on ion channels, transmembrane potentials of cardiomyocytes in vitro and heart rhythm in vivo in mice.Int J Nanomedicine. 2019 Jul 22;14:5595-5609. doi: 10.2147/IJN.S209135. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31413565 Free PMC article.
-
Molecular Mechanisms of Zinc Oxide Nanoparticle-Induced Genotoxicity Short Running Title: Genotoxicity of ZnO NPs.Materials (Basel). 2017 Dec 14;10(12):1427. doi: 10.3390/ma10121427. Materials (Basel). 2017. PMID: 29240707 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

