Defining benchmark values for outcomes of comprehensive resection of primary retroperitoneal liposarcoma: a retrospective multicenter study
- PMID: 40521170
- PMCID: PMC12167443
- DOI: 10.1016/j.eclinm.2025.103280
Defining benchmark values for outcomes of comprehensive resection of primary retroperitoneal liposarcoma: a retrospective multicenter study
Abstract
Background: Comprehensive resection represents the standard of care for patients affected by retroperitoneal well- or dedifferentiated liposarcoma (WDLPS/DDLPS). However, reference values to indicate the best achievable results are currently lacking. As such, the study aimed to define clinically relevant benchmark values for intra- and postoperative outcomes of patients undergoing comprehensive resection for primary retroperitoneal WDLPS/DDLPS.
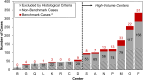
Methods: The international, prospectively maintained Retroperitoneal Sarcoma Registry (RESAR; NCT03838718) was used to calculate benchmark values for 22 outcomes, including intraoperative factors, and rates of complications, recurrence and survival. Only low-risk patients undergoing comprehensive resection for WDLPS/DDLPS at high-volume centers between 1st January 2017 and 31st December 2021 were used to calculate the benchmark values. Specifically, "low risk" was defined as age <75 years, with minimal comorbidities, and undergoing a "standard" comprehensive resection including at least colon and kidney with or without other organs-excluding those associated with significant morbidity (e.g., pancreas). Benchmark values were defined based on the 25th or 75th percentiles of the center-level data. To validate the benchmark values, these were applied to two cohorts expected to have inferior outcomes, which were defined by changing one of the exclusion criteria; namely those treated in low-volume centers, and those with American Society of Anesthesiologists (ASA) score ≥3 ("ASA ≥ 3").
Findings: Of the 1510 patients undergoing surgery, 147 met the inclusion criteria and were included in the benchmarking analysis. This identified benchmark values including: median duration of surgery ≤278 min, intraoperative packed red cell transfusion rate ≤30%, R0/R1 resection rate ≥89%, median length of hospital stay ≤15 days, reoperation rate ≤13%, major postoperative complication rate ≤21%, and 90-day postoperative mortality/failure-to-rescue rates of 0%. The "low-volume centers" cohort failed to meet 10 of these benchmarks, including duration of surgery (median: 293 vs. ≤278 min), R0/R1 resection rate (82% vs. ≥ 89%), major postoperative complication rate (35% vs. ≤21%), and reoperation rate (35% vs. ≤13%), whilst the "ASA ≥ 3" cohort failed to meet seven benchmarks.
Interpretation: These novel benchmark values can act as reference values to which sarcoma centers or individual surgeons can compare, which may help to identify performance gaps and improve the quality of care.
Funding: "5 x mille" fund for healthcare research (Italian Ministry of Health).
Keywords: Benchmark; Liposarcoma; Quality of surgery; Retroperitoneal sarcoma; Surgical oncology.
© 2025 The Author(s).
Conflict of interest statement
Piotr Rutkowski received honoraria for lectures and Advisory Boards from Bristol-Myers Squibb, MSD, Novartis, Pierre Fabre, Philogen, Genesis, Medison Pharma outside of the scope of the manuscript. His institution received research funding from Novarits, Pfizer, Roche, Bristol-Myers Squibb. Jason Sicklick serves as a consultant for CureMatch, Deciphera and Kura; received speakers’ fees from Daiichi Sankyo, Deciphera, Foundation Medicine, La-Hoffman Roche, Merck, QED, and SpringWorks; and owns stock in CureMatch and Personalis. Ferdinando Carlo Maria Cananzi received a lecture fee from Istituto Gentili. All the other authors declare no conflict of interest.
Figures

References
-
- Tirotta F., Parente A., Hodson J., et al. Cumulative burden of postoperative complications in patients undergoing surgery for primary retroperitoneal sarcoma. Ann Surg Oncol. 2021;28(12):7939–7949. - PubMed
-
- Gronchi A., Strauss D.C., Miceli R., et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. 2016;263(5):1002–1009. - PubMed
-
- Bonvalot S., Rivoire M., Castaing M., et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27(1):31–37. - PubMed
-
- Gronchi A., Lo Vullo S., Fiore M., et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27(1):24–30. - PubMed
-
- Gronchi A., Miceli R., Colombo C., et al. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol. 2012;23(4):1067–1073. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

