Boosting mRNA cancer vaccine efficacy via targeting Irg1 on macrophages in lymph nodes
- PMID: 40521193
- PMCID: PMC12159843
- DOI: 10.7150/thno.110305
Boosting mRNA cancer vaccine efficacy via targeting Irg1 on macrophages in lymph nodes
Abstract
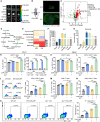
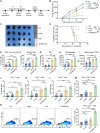
Rationale: mRNA cancer vaccines show great promise for tumor therapy, but the therapeutic efficacy is limited. Metabolites play critical roles in immunomodulation. However, their role in mRNA cancer vaccines remains poorly understood. Methods: Metabolome analysis and single-cell RNA sequence were performed to explore the most important metabolite and its source cell. B16-F10-OVA-bearing wide-type and Irg1-depleted C57BL/6 mice were treated with OVA-LNP, OVA&si-Irg1-LNP, or anti-PD-1 antibody to evaluate therapeutic efficacy. Flow cytometry analysis was used to examine the immune cells within the lymph nodes, spleens, and the tumor immune environment. Results: We found that macrophage-derived itaconate was increased markedly in activated ipsilateral lymph nodes after ovalbumin-encoding mRNA-lipid nanoparticle (OVA-LNP) injection, compared to homeostatic contralateral lymph nodes. Depleting the immune-responsive gene 1(Irg1), which encodes the itaconate-production enzyme aconitate decarboxylase (ACOD1), in macrophages improved dendritic cell antigen presentation and enhances T cell function. Combining Irg1 knockdown via small interfering RNA (siRNA) with OVA mRNA in LNPs augmented the therapeutic efficacy of mRNA cancer vaccines, both as monotherapy and in combination with an anti-programmed cell death-1 antibody. Conclusions: Our findings reveal a link between itaconate and mRNA cancer vaccines, suggesting that targeting Irg1 via siRNA-LNP could be a promising strategy to improve the therapeutic efficacy of mRNA cancer vaccines.
Keywords: DCs; anti-PD-1 antibody; mRNA cancer vaccines; macrophages; taconate.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Conry RM, LoBuglio AF, Wright M, Sumerel L, Pike MJ, Johanning F. et al. Characterization of a messenger RNA polynucleotide vaccine. Cancer Res. 1995;55:1397–400. - PubMed
-
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–80. - PubMed
-
- Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

