BCAA catabolism targeted therapy for heart failure with preserved ejection fraction
- PMID: 40521197
- PMCID: PMC12159833
- DOI: 10.7150/thno.105894
BCAA catabolism targeted therapy for heart failure with preserved ejection fraction
Abstract
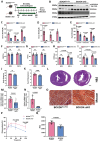
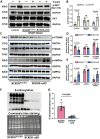
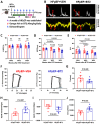
Rationale: Heart failure with preserved ejection fraction (HFpEF) is a major unmet medical need with limited effective treatments. A significant contributing factor to HFpEF, a multifactorial disease, is underlying metabolic dysfunction. While much of the prior research has been on glucose and fatty acid metabolic defects in the pathogenesis of HFpEF, other metabolic activities remain under investigated. Methods: System-based metabolomics and targeted mass spectrometry were employed to analyze serum and tissue samples from a deep-phenotyped human HFpEF cohort. A preclinical mouse model of HFpEF was developed by combined administration of a high-fat diet (HFD) and the nitric oxide (NO) synthase inhibitor N[w]-nitro-l-arginine methyl ester (L-NAME). The branched-chain amino acid (BCAA) catabolic activities were enhanced by genetic inactivation of branched-chain ketoacid-dehydrogenase kinase (BCKDK) or treatment with BT2 (3,6-dichlorobenzo[b]thiophene-2-carboxylic acid), a highly selective inhibitor of BCKDK. Cardiac function, myocardial remodeling and insulin signaling in the left ventricle were assessed across all experimental cohorts. Results: The systems-based metabolomics analysis of the deep-phenotyped HFpEF and non-HFpEF patients revealed that abnormal circulating BCAA levels were significantly associated with adverse outcomes. In the rodent model of HFpEF, significant impairment of BCAA catabolic activities in the heart and abnormal circulating BCAA levels were also observed. In adult mice, inducible knockout of BCKDK, the rate-limiting negative regulator of BCAA catabolic flux, markedly augmented BCAA catabolic activities. Compared with the controls, BCKDK inactivation blunted diastolic dysfunction, cardiac hypertrophy and myocardial remodeling in response to chronic treatment with HFD/L-NAME. This functional amelioration was associated with improved insulin signaling in the myocardium and reduced S-nitrosylation of cardiac proteins, without any impact on systemic blood pressure. Finally, pharmacological inhibition of BCKDK in HFpEF mice significantly reversed the diastolic dysfunction and cardiac hypertrophy associated with HFpEF. Conclusions: Our study provides the first proof-of-concept evidence that global catabolic impairment of BCAAs is an important pathogenic contributor and metabolic signature of HFpEF and restoring BCAA catabolic flux could be an efficacious therapeutic strategy for HFpEF.
Keywords: BCAA metabolism; BT2; HFpEF; heart failure; insulin resistance.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–15. - PubMed
-
- Redfield MM, Borlaug BA. Heart Failure with Preserved Ejection Fraction: A Review. JAMA. 2023;329:827–838. - PubMed
-
- Ketema E, Lopaschuk GD. The impact of obesity on cardiac energy metabolism and efficiency in heart failure with preserved ejection fraction. Can J Cardiol. 2025;30:S0828–282X. (25)00099-6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

