Challenges and opportunities for the diverse substrates of SPOP E3 ubiquitin ligase in cancer
- PMID: 40521202
- PMCID: PMC12159753
- DOI: 10.7150/thno.113356
Challenges and opportunities for the diverse substrates of SPOP E3 ubiquitin ligase in cancer
Abstract
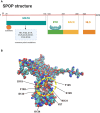
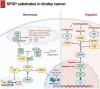
The Speckle-type POZ protein (SPOP), a substrate adaptor of the cullin-RING E3 ligase complex, mediates both the degradation and non-degradative ubiquitination of substrates, which are crucial for regulating various biological functions and cellular processes. Dysregulation of SPOP-mediated ubiquitination has been implicated in several cancers. Emerging evidence suggests that SPOP functions as a double-edged sword: acting as a tumor suppressor in prostate cancer (PCa), hepatocellular carcinoma (HCC), and colorectal cancer (CRC), while potentially serving as an oncoprotein in kidney cancer (KC). Therefore, SPOP's role in tumorigenesis appears to be tissue- or context-dependent. Numerous downstream substrates of SPOP have been identified across various cancers, where they regulate carcinogenesis, metabolic reprogramming, cell death, immune evasion, therapy resistance, and tumor microenvironment (TME) remodeling. However, the definitive role of SPOP in these cancers requires further investigation. A comprehensive understanding of the molecular mechanisms of SPOP in different cancer types will provide new insights into its function in oncogenesis, potentially advancing anti-cancer drug development. Here, we summarize the latest findings on SPOP's functions and structural features, its regulatory mechanisms, the roles of its substrates in various cancers, and SPOP-targeting strategies.
Keywords: SPOP; cancer; diverse substrates; functions; therapeutic targeting.
© The author(s).
Conflict of interest statement
Competing Interests: All authors declare no conflict of interest.
Figures

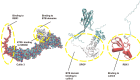





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

