Loss of SLC25A20 in Pancreatic Adenocarcinoma Reversed the Tumor-Promoting Effects of a High-Fat Diet
- PMID: 40521210
- PMCID: PMC12160018
- DOI: 10.7150/thno.114912
Loss of SLC25A20 in Pancreatic Adenocarcinoma Reversed the Tumor-Promoting Effects of a High-Fat Diet
Abstract
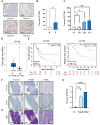
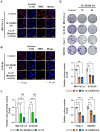
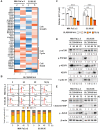
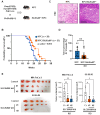
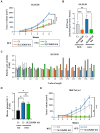
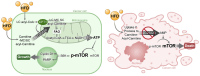
Rationale: Although it is known that High-fat diet (HFD) promotes the development of pancreatic ductal adenocarcinoma (PDAC), no direct link between HFD and cancer has been identified. Previously, we showed that ATP production by cancer cells depends on fatty acid oxidation (FAO); therefore, we hypothesized that blocking FAO may prevent HFD-induced promotion of PDAC growth. Methods: To determine whether FAO is increased in PDAC patients, we analyzed a tissue microarray by immunohistochemical staining to detect carnitine palmitoyl transferase I. To block FAO, SLC25A20 (carnitine-acylcarnitine carrier) was knocked down in cancer cells, which was implanted for xenograft in mice and treated with a high-fat diet (HFD, 60% fat). To compare cancer development including survival rates, and histopathological differences were analyzed by crossbreeding of KPC mice (KrasG12D/+; Trp53R172H/+; Pdx1-Cre) with KPC/Slc25a20+/- mice. Results: SLC25A20 knockdown in cancer cells reduced ATP production and inhibited cell growth. Proteome analysis revealed that SLC25A20 knockdown reduced cancer cell growth significantly due to inactivation of mTOR via decreased ATP production, ultimately leading to cell death. The median survival time of KPC/Slc25a20+/- tumor-bearing mice was 3.1 weeks longer than that of KPC tumor-bearing mice. In mice fed an HFD, the growth of xenografts derived from SLC25A20 knockdown PDAC cells was 65-95% lower than that of xenografts derived from control cells. Conclusion: Blocking FAO by SLC25A20 knockdown reversed HFD-induced promotion of PDAC growth.
Keywords: Fatty acid oxidation; High-fat diet; PDAC; Pancreatic cancer; SLC25A20.
© The author(s).
Conflict of interest statement
Competing Interests: The authors, except SK, declare no conflicts of interest. SK has filed a patent to develop an anti-cancer drug targeting SLC25A20 and founded an in-house venture company at the National Cancer Center in the Republic of Korea.
Figures


References
-
- An C, Pipia I, Ruiz AS, Arguelles I, An M, Wase S. et al. The molecular link between obesity and genomic instability in cancer development. Cancer Lett. 2023;555:216035. - PubMed
-
- Rawla P, Thandra KC, Sunkara T. Pancreatic cancer and obesity: epidemiology, mechanism, and preventive strategies. Clin J Gastroenterol. 2019;12:285–91. - PubMed
-
- Molina-Montes E, Coscia C, Gomez-Rubio P, Fernandez A, Boenink R, Rava M. et al. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut. 2021;70:319–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

