Structural Adaptations of Bacterial Grx3 to Temperature: Pro29 Is Essential for Cold Adaptation in Sphingomonas sp. Grx3
- PMID: 40521461
- PMCID: PMC12163845
- DOI: 10.1021/acsomega.5c03414
Structural Adaptations of Bacterial Grx3 to Temperature: Pro29 Is Essential for Cold Adaptation in Sphingomonas sp. Grx3
Abstract
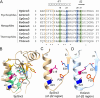
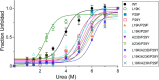
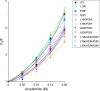
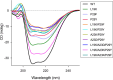
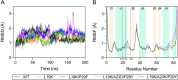
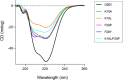
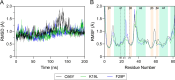
Bacterial glutaredoxin 3 (Grx3) is a class I oxidoreductase with a canonical thioredoxin (Trx) fold, yet its thermal adaptation mechanisms remain unclear. We investigated cold adaptation in the psychrophilic ortholog from the Arctic bacterium Sphingomonas sp. (SpGrx3). While mesophilic orthologs, such as Escherichia coli Grx3 (EcGrx3), typically feature an α1-β2 salt bridge (Lys19-Glu31), SpGrx3 lacks this bridge due to Leu19, although Glu31 is conserved. The L19K mutation in SpGrx3 failed to form the salt bridge, yielding the least stable mutant. However, the introduction of a P29F or P29Y substitution in the α1 loop in combination with L19K restored the salt bridge and significantly enhanced both the melting temperature and stability. Phe29 stabilizes the structure via hydrophobic interactions, enhancing substrate affinity, while Tyr29 enhances catalytic rates through hydrogen bonding. Conversely, the reciprocal F29P substitution in EcGrx3 disrupted the salt bridge and markedly reduced its melting temperature. Notably, K19L/F29P, which mimics the SpGrx3 wild-type (WT) configuration, increased melting temperature via Leu19 hydrophobic interactions, similar to F29Y with the salt bridge. These results underscore the crucial role of Phe or Tyr at position 29 in forming the Lys19-Glu31 salt bridge in warmer-temperature orthologs and suggest that the transition to Pro is a critical adaptation in psychrophilic orthologs. This study provides new insights into Grx3's structural adaptations to varying thermal habitats through diverse α1-β2 interactions.
© 2025 The Authors. Published by American Chemical Society.
Figures

References
LinkOut - more resources
Full Text Sources
